ISO 13485 Certification India
Are you looking for ISO 13485 certification as a Medical Device Manufacturer? Pharmadocx Consultants is here to help you! As a leader in ISO certification industry, we will ensure you get ISO 13485 certification. Let our experienced consultants navigate you through the intricacies of certification, ensuring you meet and surpass global benchmarks.
What is ISO 13485?
ISO 13485 is an international standard that specifies requirements for a quality management system (QMS) for organizations involved in the medical device industry. It is designed to ensure that organizations can consistently meet both customer and regulatory requirements applicable to medical devices and related services.

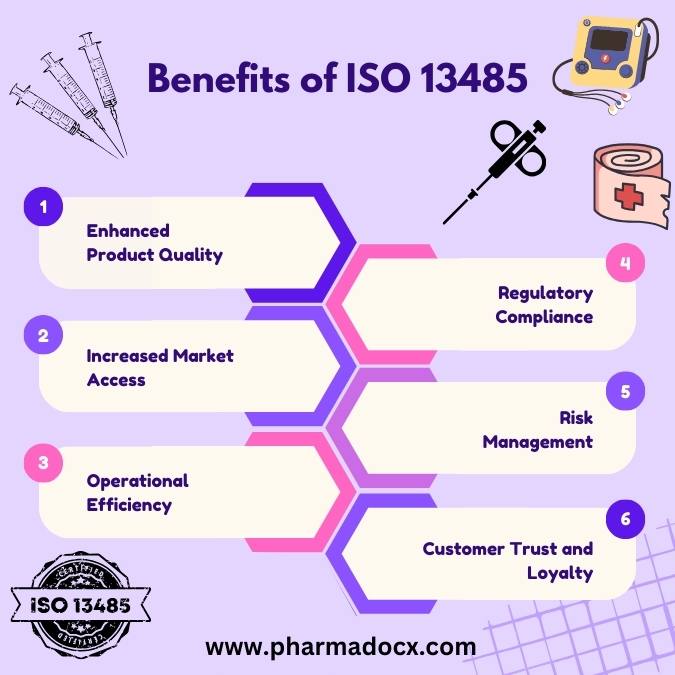
Benefits of ISO 13485
- Enhanced Product Quality: ISO 13485 focuses on quality management processes, ensuring consistent and high-quality medical devices.
- Regulatory Compliance: Many countries require medical device manufacturers to be ISO 13485 certified, or they use the standard as a basis for their regulations. Achieving this certification can simplify regulatory compliance and facilitate market entry.
- Increased Market Access: ISO 13485 certification can be a prerequisite for doing business in certain countries or with specific customers. Certification can open doors to global markets.
- Risk Management: The standard emphasizes risk management throughout the product lifecycle, leading to safer products and reduced liability for the manufacturer.
- Operational Efficiency: Implementing the practices and processes of ISO 13485 can streamline operations, reduce errors and rework, and lead to cost savings.
- Customer Trust and Loyalty: Being ISO 13485 certified can boost the reputation of an organization, signaling to customers and stakeholders that the company is committed to quality and safety.
ISO 13485 Clauses
ISO 13485 is structured into several clauses, each addressing a specific aspect of the quality management system for medical devices. They are:
- Scope: Defines the applicability and boundaries of the standard, focusing on the requirements for a comprehensive quality management system for medical device manufacturing.
- Normative References: Lists the reference documents essential for the application of ISO 13485.
- Terms and Definitions: Provides definitions of specific terms and concepts used throughout the standard.
- Quality Management System: Describes the general requirements for establishing, maintaining, and improving the quality management system.
- Management Responsibility: Outlines the roles and responsibilities of management in supporting and maintaining the quality management system.
- Resource Management: Details the provisions for adequate resources, including human resources, environment for product processes, and training.
- Product Realization: Specifies the processes and controls necessary from design and development to delivery of the product.
- Measurement, Analysis, and Improvement: Addresses the monitoring and measurement of processes, non-conformities, corrective actions, and continual improvement of the quality management system.
How to get ISO 13485 Certification?
- Awareness and Training: Familiarize yourself with the ISO 13485 standard and its requirements, possibly through training courses or seminars.
- Gap Analysis: Review your current processes and systems against the ISO 13485 requirements to identify gaps or areas of improvement.
- Documentation Development: Develop or update your Quality Management System (QMS) documentation, including policies, procedures, and work instructions.
- Implementation: Put the processes and systems outlined in your documentation into practice across the organization.
- Internal Audit: Conduct an internal audit to check your QMS’s effectiveness and ensure compliance with ISO 13485.
- Management Review: Senior management reviews the QMS to ensure its continuing suitability, adequacy, and effectiveness.
- Select a Certification Body: Choose an accredited certification body (auditor) to perform the external audit.
- Audits: The certification body reviews your QMS documentation and verifies your readiness for the full certification audit. If the auditor identifies any non-conformities, correct them and provide evidence of the corrections to the auditor.
- Obtain Certification: Once you pass the audit and address any non-conformities, the certification body will issue your ISO 13485 certificate.
- Ongoing Maintenance: Conduct regular internal audits, and management reviews, and address any non-conformities to ensure continued compliance. Periodic reviews by the certification body (typically annually) to ensure continued adherence to the standard.
How Pharmadocx Consultants can help you with ISO 13485 certification?
We have been helping Medical Device Manufacturers get ISO 13485 certification since 2007. Our team of experts makes sure you comply with all the clauses of ISO 13485 and ensure a successful grant of the certification. As one of India’s premier ISO 13485 certification consultants, Pharmadocx Consultants brings to the table a blend of experience and expertise. Allow us to partner with you, ensuring a seamless certification process and a brighter global footprint.
Gap Analysis
We’ll assess your current systems, identifying areas that need enhancement to meet ISO 13485 standards.
Tailored Solutions
Every business is unique. We provide customized strategies for ISO 13485 compliance, fitting your specific needs.
Expert Guidance
Our seasoned professionals break down the ISO 13485 requirements, ensuring you understand every detail.
Training and Workshops
We provide hands-on training sessions and workshops for your team, ensuring everyone is equipped with the knowledge and skills needed to uphold ISO 13485 standards consistently.
Audit Preparation
With our assistance, you’ll be well-prepared for both internal and external audits, ensuring a smooth certification process.
Successful Grant of ISO 13485
We ensure you comply with all clauses of ISO 13485 and get the certification. Even after certification, we’re here for you, offering support for maintenance, renewals, and updates to the standard.
Let's Talk!
We'd love to hear from you! Whether you have questions about our pharmaceutical plant setup consultation services or want to discuss a potential project, our team is here to help. Simply fill out the form below, and we'll get back to you as soon as possible. Alternatively, you can reach out to us directly using the phone number or email address listed on this page. We look forward to connecting with you!
Phone / Whatsapp
Address
- Sonipat Office - Opposite Dewan Mill, Old D.C. Road Sonepat - 131001 Haryana, India
- Delhi Office - G-12, Pearls Best Heights-I, Netaji Subhash Place, Delhi, 110034
Frequently Asked Questions (FAQs)
What is ISO 13485?
ISO 13485 is an international standard that outlines requirements for a quality management system (QMS) specific to the medical device industry. It ensures that organizations can consistently meet customer and regulatory requirements for medical device production and related services.
What is the difference between ISO 13485 and 21 CFR 820?
ISO 13485 is a global standard for quality management systems in the medical device industry. In contrast, 21 CFR 820, also known as the Quality System Regulation (QSR), is specific to the United States and is enforced by the U.S. Food and Drug Administration (FDA). While both standards aim to ensure the safety and efficacy of medical devices, they have different requirements and scopes.
What is the purpose of ISO 13485?
The primary purpose of ISO 13485 is to facilitate harmonized medical device regulatory requirements for quality management systems. It provides a framework for companies to ensure consistent design, development, production, installation, and delivery of medical devices that are safe for their intended purpose.
Who needs ISO 13485 certification?
Manufacturers of medical devices, suppliers, and service providers in the medical device industry should consider ISO 13485 certification. It’s often a requirement for doing business in certain countries or regions and provides a competitive advantage in the global marketplace.
What is the ISO 13485 quality management system?
The ISO 13485 quality management system (QMS) is a comprehensive set of policies, processes, and procedures required for the design, development, production, and delivery of medical devices. It emphasizes risk management, regulatory compliance, and continuous improvement to ensure the safety and effectiveness of devices.
When is the latest release of ISO 13485?
The latest release of ISO 13485 is ISO 13485:2016. It was published in March 2016 and introduced several updates and changes to address the evolving needs and challenges of the medical device industry.


