CDSCO Import License Consultant for Medical Devices in India
Introduction to CDSCO Import Licensing for Medical Devices in India
When it comes to importing medical devices into India, the Central Drugs Standard Control Organisation (CDSCO) is the key regulatory authority. Every medical device entering the Indian market must comply with the regulations set by CDSCO, ensuring the safety and efficacy of the product. CDSCO is responsible for setting the standards for drugs and medical devices in India. They control the quality of imported devices and coordinate with State Drug Control Organizations. Their primary goal is to ensure that every medical device available to the Indian population meets stringent safety standards.
Recent Changes in CDSCO’s Guidelines
As per CDSCO’s new guidance, without an import license, a company cannot import Class A, B, C, and D medical devices after the specified deadline for the mandatory Import license (MD 15). This change underscores the importance of timely compliance with CDSCO regulations. Previously, manufacturers could sell their devices in India without many regulations. However, since 2017, a shift occurred. Now, medical devices entering India must adhere to specific import guidelines set by CDSCO. This procedure applies to all importers and distributors bringing medical devices from other countries into India. Moreover, every device needs classification as per CDSCO’s rules.
In essence, if you’re looking to import medical devices into India, understanding and complying with CDSCO’s regulations and getting an import license is not just recommended; it’s mandatory. Official Circular Link
Looking for CDSCO Import License for Medical Devices?
Contact us today to start your journey in the world of Medical Devices!
CDSCO Medical Device Import License Overview
Document Required for the Registration Process:
- Manufacturing License
- Free Sale Certificate
- Free Sale Certificate (From EU, USA, Australia, Canada, Japan)
- ISO 13485:2016
- CE Certificate
- CE Design Certificate
- Declaration of Conformity
- Audit report of the premises
- Device Master File as per Medical Device Rules, 2017
- Plant Master File as per Medical Device Rules, 2017
Contents of a Device Master File (DMF):
- Administrative Information: Key contact details and table of contents.
- Device Description: Overview of device and its intended use.
- Materials: List of materials, sources, and quality specifications.
- Manufacturing: Process flow, equipment details, and validation.
- Quality Control: Testing procedures and acceptance criteria.
- Sterilization: Method details and validation.
- Packaging and Labeling: Packaging components and labeling artwork.
- Performance Testing: Bench and in-vivo/in-vitro test summaries.
- Safety Testing: Risk analysis and safety test results.
- Clinical Information: Clinical study design and outcomes.
Read more about Device Master Files
CDSCO Medical Device Import License Registration
In India, the CDSCO governs the import license for medical devices. Industries or individuals possessing a wholesale license (Form 20/21 B) or a registration certificate (Form 41/42) are eligible to apply for the CDSCO import license under the Medical Device Rules, 2017. This license permits the import of various medical devices into the country.
For foreign manufacturers aiming to enter the Indian market, it’s essential to designate an authorized agent or importer within India to facilitate the medical device registration. The necessary documentation for obtaining the CDSCO import license has been outlined in recent regulatory updates. Once granted, the CDSCO import license remains valid for a period of five years. Furthermore, according to CDSCO guidelines, foreign manufacturers must provide specific regulatory certificates as a prerequisite for registering medical devices in India.

Steps of CDSCO Medical Device Import License Registration
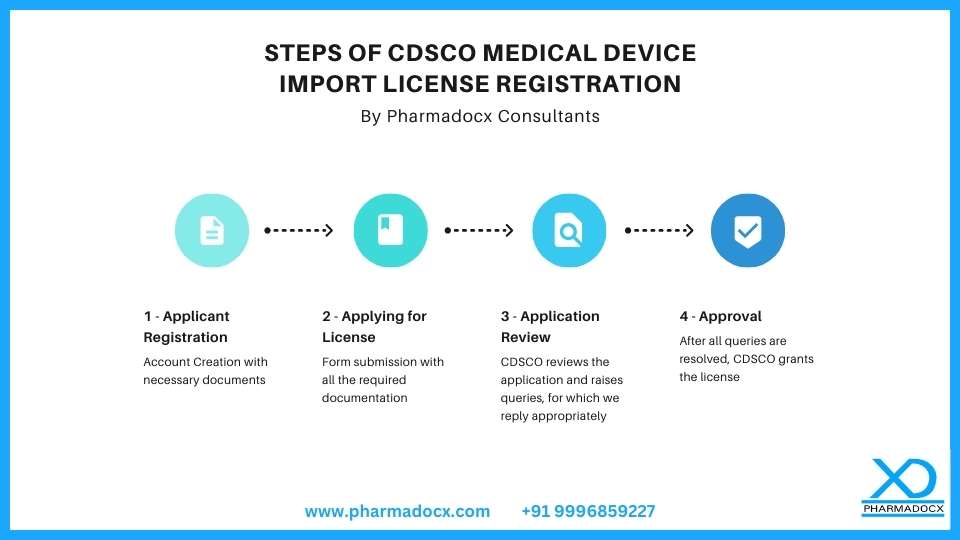
Step 1 – Applicant Registration
- Getting Started: Before diving into the application, ensure you have an active account on the CDSCO online registration portal.
- Who Can Apply?: An authorized agent or distributor with a valid wholesale drug license (form 20/21B) or a registration certificate (form 41/42) is eligible to apply.
- Application Submission: Use Form MD-14 on the CDSCO portal to initiate the process.
- Document Upload: Submit the necessary documents online.
- Awaiting Feedback: CDSCO will review the application. If there are issues, they’ll communicate the reasons, and you might need to reapply.
Step 2 – Diving Deeper: Medical Device Import License Application
- Form Details: The online form will require specifics like the device’s classification, brand name, intended use, and a detailed product description.
- Document Submission: Upload essential documents in line with Indian CDSCO regulations. This might include regulatory certificates like European CE, free sale certificate, ISO 13485, Plant Master File, and Device Master File.
- Fee Payment: Pay the CDSCO fee, which varies based on the device’s classification.
- Confirmation: Once all documents are submitted and fees are paid, you’ll receive an application number, confirming successful submission.
Step 3 – Application Review by CDSCO
- Thorough Examination: CDSCO will meticulously review the application and all attached documents.
- Open Communication: If there are queries or discrepancies, CDSCO will reach out for clarification.
- Your Response: It’s crucial to promptly submit any requested justifications or revised documents through the CDSCO online portal.
Step 4 – License Approval
- Final Review: CDSCO will once again scrutinize the justifications and documents. If everything aligns with their regulations, they’ll move forward.
- License Grant: Meeting all criteria and addressing all queries will result in the granting of the import license for your medical device.
Why Choose Pharmadocx Consultants for Your CDSCO Import License Needs?
Medical Device MD-5, MD-9 Licences
Plants Set-up
Years Experience
- Expertise in CDSCO Regulations: Our team is well-versed with the nuances of CDSCO guidelines and the Indian Medical Device Regulation. We ensure that your application aligns perfectly with the regulatory requirements, increasing the chances of swift approval.
- End-to-End Assistance: From initiating the applicant registration to celebrating the license approval, we’re with you at every step. Our comprehensive service ensures that no detail, however minute, is overlooked.
- Time-Efficient Processes: We understand the value of time in the medical device industry. Our streamlined processes, backed by years of experience, ensure that your application is processed in the shortest time frame possible.
- Document Preparation and Review: With our in-depth knowledge of the required documentation, from Plant Master Files to Device Master Files, we not only assist in preparing these documents but also review them to ensure accuracy and completeness.
- Regular Updates: The waiting period can be stressful. We provide regular updates on the status of your application, ensuring you’re always in the loop.
- Post-License Support: Our relationship doesn’t end with the license approval. We offer post-license support, assisting with renewals and any regulatory changes that might affect your business.
- Cost-Effective Solutions: Quality doesn’t always have to come at a high price. Our competitive pricing ensures you get the best services without burning a hole in your pocket.
- Client-Centric Approach: At Pharmadocx Consultants, our clients are our top priority. We tailor our services to meet your specific needs, ensuring a personalized experience.
In the ever-evolving landscape of medical device regulations in India, having a trusted partner can make all the difference. Let Pharmadocx Consultants be that partner, guiding you towards success in the Indian medical device market. Reach out to us today and take the first step towards hassle-free CDSCO import licensing.
Call us at 9996859227 or write to us at [email protected]
Let's Talk!
We'd love to hear from you! Whether you have questions about our pharmaceutical plant setup consultation services or want to discuss a potential project, our team is here to help. Simply fill out the form below, and we'll get back to you as soon as possible. Alternatively, you can reach out to us directly using the phone number or email address listed on this page. We look forward to connecting with you!
Phone / Whatsapp
Address
- Sonipat Office - Opposite Dewan Mill, Old D.C. Road Sonepat - 131001 Haryana, India
- Delhi Office - G-12, Pearls Best Heights-I, Netaji Subhash Place, Delhi, 110034
Pharmadocx Consultants offers services across India!
CDSCO Import License for Medical Devices in Ahmedabad
Frequently Asked Questions (FAQs)
What is CDSCO and its role in medical device import licensing?
CDSCO stands for Central Drugs Standard Control Organization. It’s the primary regulatory body in India overseeing the safety, efficacy, and quality of drugs, cosmetics, and medical devices. It’s responsible for granting import licenses for medical devices.
How long is the CDSCO import license for medical devices valid?
The CDSCO import license for medical devices is typically valid for 5 years from the date of issuance and then needs to be applied for retention.
What are the different phases involved in the CDSCO import license registration process?
The process involves four main phases: Applicant Registration, Import License Application, CDSCO Review, and License Approval. At Pharmadocx Consultants, we guide clients through each phase seamlessly.
What documents are required for CDSCO medical device import licensing?
Key documents include regulatory certificates like Free Sale Certificate, ISO 13485, Plant Master File, and Device Master File. Our team at Pharmadocx Consultants ensures all documents are prepared and submitted correctly.
How does the classification of medical devices by CDSCO impact the licensing process?
CDSCO classifies medical devices based on risk. The classification determines the documentation and fees required.
Are there any specific guidelines for foreign manufacturers to obtain a CDSCO import license?
Yes, foreign manufacturers must appoint an authorized agent or distributor in India to register their medical devices.
How long does it typically take to get a CDSCO import license for medical devices?
Usually it takes 2-3 months to get CDSCO Import License for Medical Devices. The duration varies based on the device’s classification and documentation. With our 27 years of experience, Pharmadocx Consultants can expedite the process for you.
What are the fees associated with the CDSCO import license application?
The fees varies for each risk category for Medical Devices CDSCO Import License.
| Risk Class | Class A | Class B | Class C&D |
| Per Site | $1,000 | $2,000 | $3,000 |
| Per Product | $50 | $1,000 | $1,500 |
What is form MD-14 in CDSCO?
Form MD-14 is the application form for obtaining an import license for medical devices from CDSCO.
Where to apply for a medical device import license?
Applications are submitted through the CDSCO online portal. Let Pharmadocx Consultants handle the intricacies for you.
What is the cost of an Import License for a medical device in India?
The fees varies for each risk category for Medical Devices CDSCO Import License.
| Risk Class | Class A | Class B | Class C&D |
| Per Site | $1,000 | $2,000 | $3,000 |
| Per Product | $50 | $1,000 | $1,500 |
Can I transfer my CDSCO import license to another entity or individual?
No you can’t transfer a CDSCO Import License for Medical Devices to anyone else.
What is a CDSCO MD-15 License?
The MD-15 license is a specific authorization issued by CDSCO for the import of particular medical devices into India, ensuring they meet the necessary safety and quality standards.

