Easily obtain GMP certificate as per Schedule M in India
The Indian regulatory body for pharmaceuticals wants to increase the accountability of drug manufacturers for patient safety. The aim is to make India a global pharma manufacturing hub. Good manufacturing practices (GMP) in pharma industry regulate all aspects of the drug-production process for improved patient safety and outcome. Furthermore, with the vision of making India a global pharma manufacturing hub, Schedule M is being strictly implemented. The goal is to bring the Indian pharmaceutical regulations at par with international standards. Thus, securing GMP Certificate as per Schedule M is becoming increasingly important for pharma companies to function in India.
Pharmadocx Consultants GMP certificate services
The Pharmadocx Consultants team is aimed at streamlining the complex process of securing GMP Certificate as per Schedule M.
We will leverage our Schedule M guideline expertise to ensure your pharma company complies with Schedule M GMP certificate requirements.
The Pharmadocx Consultants team has extensive knowledge of the Schedule M GMP guidelines, regulations, and requirements.
We will provide holistic support to help you easily achieve Schedule M GMP compliance.
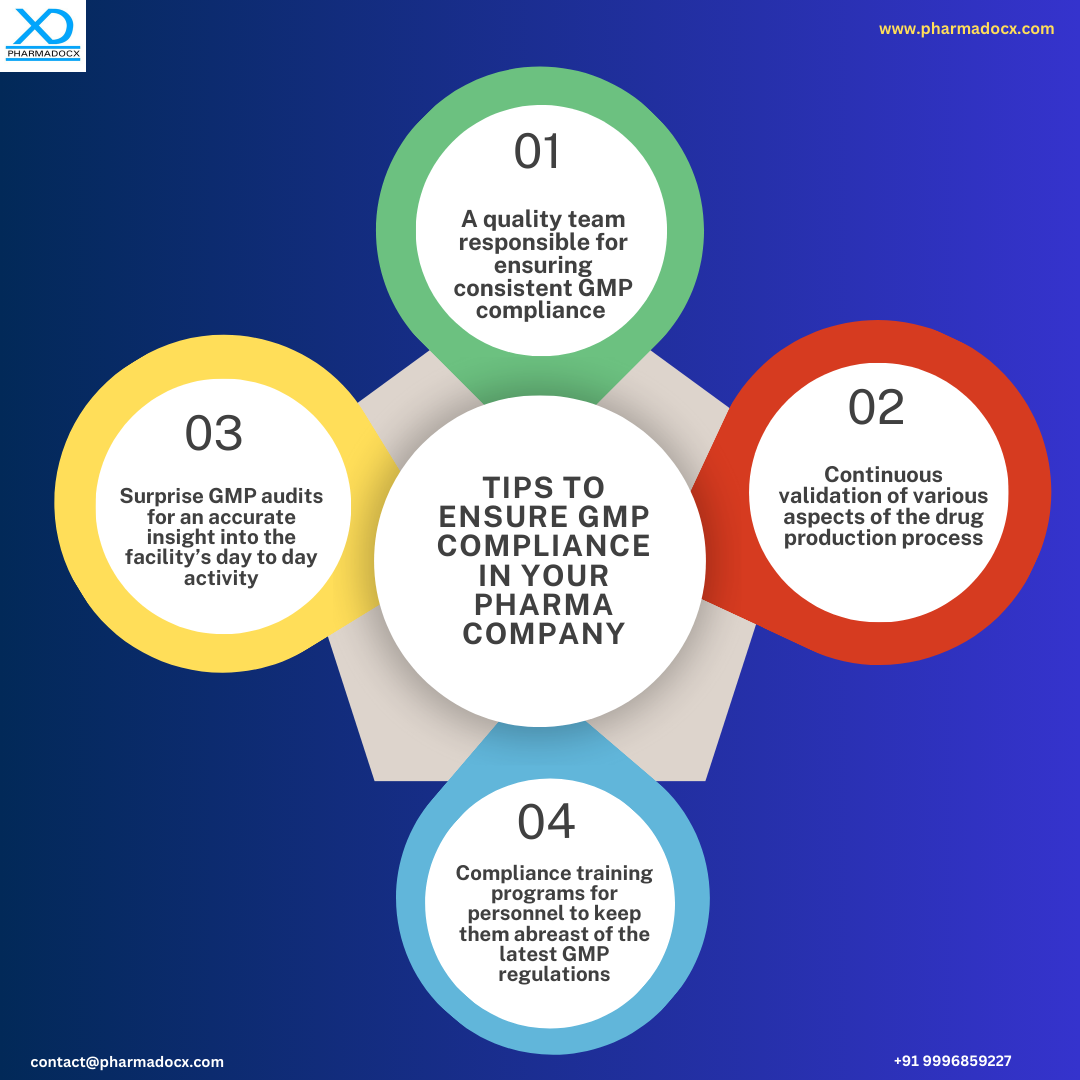
What is GMP or good manufacturing practices?
Good manufacturing practices (GMP) is a system that sets guidelines for consistently manufacturing products as per defined quality benchmarks. GMP covers every aspect of the manufacturing process to prevent cross-contamination, adulteration, and mislabelling. Pharma GMP guidelines are aimed at protecting patients from health hazards by regulating the quality, efficacy, and safety of pharmaceuticals.
GMP guidelines are developed to minimize pharmaceutical production associated risks that cannot be eliminated only by testing the finished product. Some of the risks in pharmaceutical production are as follows: unexpected cross-contamination or adulteration of drugs, incorrect labelling of containers, and addition of insufficient or too much of active ingredients. Good manufacturing practices provide detailed written guidelines for each step in the drug production process that could affect the quality of the final product. Hence, a GMP certificate is issued only if the drugs are manufactured per GMP guidelines.
Implementing GMP in your production process can help reduce losses and waste. Additionally, you can avoid product recalls, fines, and legal action for manufacturing substandard products. Thus, good manufacturing practices in pharma industry protects both patients and the company.
Aspects of the pharma industry governed by GMP certificate requirements
GMP guidelines and requirements regulate all aspects of pharma industry that will impact the safety and quality of manufactured drugs. They cover all areas of production from raw materials, facilities, and equipment to staff training and personal hygiene. Aspects of drug production regulated are as follows:
- Manufacturing building, environment, and facilities
- Manufacturing equipment and instrument
- Raw materials
- Personnel
- Quality management
- Staff sanitation and hygiene
- Inspections and quality audits
- Product complaints
- Documentation and recordkeeping
- Validation and qualification
Mandatory Schedule M compliance for manufacturing pharmaceuticals in India: Schedule M GMP certificate
What is Schedule M?
Schedule M is a part of Drugs and Cosmetics Act 1940. It specifies the Good Manufacturing Practices for pharmaceuticals. All pharmaceutical manufacturing units in India need to comply with the Schedule M.
Strict compliance with Schedule M guidelines is required to ensure the drugs perform as intended. Hence, drug manufacturers will have to mandatorily secure GMP certificate as per Schedule M.
What is the revised Schedule M?
The government aimed to revise the Schedule M to bring the Indian pharmaceutical regulations at par with global standards. Thus, drugs manufactured in India would be globally accepted.
In the revised Schedule M, the government has placed special emphasis on manufacturing premises, plant, and equipment in addition to the existing GMP requirements. The Schedule M is aimed at safeguarding patients and improving the Indian pharmaceutical product’s reputation. The revised Schedule M specifically stresses on qualification and validation of equipment, risk management, and self-inspection.
Only after securing the GMP certificate as per Schedule M can manufacturers sell their drugs in India.
Existing pharma companies will also have to comply with the revised Schedule M guidelines
In case you have an existing factory, we will help renovate the factory as per the revised Schedule M guidelines. We will revamp your factory as per new updated guidelines at minimal expenditure with minimum operational disruption.
Our team of experts will completely modify your factory within the deadline so that you do not face legal consequences.
Why do pharma companies in India need to comply with the Schedule M?
- Compliance with Schedule M would indicate high-quality, safe, and effective drugs are being manufactured.
- Companies having GMP certificate as per Schedule M would indicate commitment to enhanced patient outcome and safety.
- Pharma companies will aim to continuously improve their pharmaceutical manufacturing process to deliver high quality products.
- Schedule M compliance would indicate compliance with most global pharmaceutical regulatory standards.
- Companies will have to adopt efficient quality management systems.
- Complying with Schedule M guidelines is now a mandatory requirement for manufacturing pharmaceuticals in India. Non-compliance can attract fines and penalties as well as regulatory sanctions.
- Pharma companies with GMP Certificate as per Schedule M are only allowed to participate in government tenders.
- Customers will prefer GMP compliant pharma manufacturers over those that are not. Healthcare professionals and patients will have increased confidence in drugs manufactured by Schedule M complaint companies.
To easily secure the GMP certificate as per Schedule M, drop us a message
The government is strictly implementing Schedule M so that drugs manufactured in India would be globally accepted. Hence, pharma companies in India should comply with Schedule M GMP guidelines to ensure the drugs manufactured in India are at par with those manufactured internationally.
Easily obtain Schedule M GMP certificate with Pharmadocx Consultants team’s help
Regulatory compliance
We will help you ensure that your manufacturing facility meets Revised Schedule M and GMP regulatory standards and guidelines. Moreover, we will ensure compliance with all the clauses of Schedule M. Furthermore, our team will thoroughly examine your facility to understand your current regulatory compliance status. Additionally, we will provide a detailed checklist for your easy reference.
Document preparation support
We will help you prepare all the documents required for compliance with Schedule M and GMP guidelines. Our team will assist in preparing and organizing all necessary SOPs and procedural documents required for securing the GMP certificate.
Staff training sessions
We will train your staff to equip them with the necessary knowledge on the GMP and Schedule M guidelines. Our mentors will empower your staff to operate your manufacturing facility per GMP guidelines. Our comprehensive training session will make your technical staff adept at handling manufacturing facility inspections and audits.
Modification of existing manufacturing facilities per latest guidelines
Based on our findings from the facility inspection, our team will prepare a detailed report on regulatory shortcomings. Then, we will revamp and renovate your existing manufacturing facility as per new updated Schedule M and GMP guidelines. We will provide precise and strategic guidance on necessary modifications for your plant. Moreover, our guidance focuses on optimizing the facility’s operational workflows, layout, equipment, and machinery.
Mock audit
Our team will conduct thorough mock audits at your manufacturing facility. We not only identify the shortcomings but also provide corrective actionable next steps. We will work relentlessly to make your manufacturing facility audit ready.
Filing for GMP certificate
Applying for the GMP certificate and filling out the application is difficult. However, our team will handle all the nitty-gritties of the application process on your behalf. We will help fill out the application form and ensure all the necessary criteria are fulfilled for a successful application.
Why choose Pharmadocx Consultants?
Drugs Licences
Years Experience
Plants Set-up
Our clients

Our GMP certification service support extends up to successfully acquiring the certificate. Our team will provide guidance on last-minute checks, compliance reviews, and final documentation. Additionally, we will act as a liaison between you and the regulatory authorities for smooth communication. Moreover, we will help interpret regulatory feedback and queries and assist in preparing responses. Thus, we will provide full support till you successfully secure the Schedule M GMP certificate.
Let's Talk!
We'd love to hear from you! Whether you have questions about our pharmaceutical plant setup consultation services or want to discuss a potential project, our team is here to help. Simply fill out the form below, and we'll get back to you as soon as possible. Alternatively, you can reach out to us directly using the phone number or email address listed on this page. We look forward to connecting with you!
Phone / Whatsapp
Address
- Head Office - Opposite Dewan Mill, Old D.C. Road Sonepat - 131001 Haryana, India
- Registered Office - Netaji Subhash Place, Delhi, 110034