Top US FDA 510(k) Consultant for Medical Devices & IVDs
Accelerate your US FDA 510(k) clearance with our support and strategic guidance.
Our US FDA 510(k) experts will help you streamline submissions, avoid costly delays, and ensure regulatory success.
Expert consultation with industry leaders
End‑to‑end 510(k) submission support
Proven expertise in US FDA guidelines for medical devices & IVDs
Documentation that passes scrutiny the first time
Accelerated US FDA 510(k) clearance
600+ Granted Approvals 27+ Years Experience
300+ Happy Clients












In the United States, the medical devices and IVDs are stringently regulated to protect the public health. Patient safety and outcome depends on the quality and effectiveness of the medical device and IVDs. Hence, rigorous regulatory standards have been set for medical devices and in vitro diagnostic products. The US FDA 510(k) approval process for medical devices and IVDs authorizes the marketing of these devices in the US. Through this process the Food and Drug Administration (FDA) evaluates device safety and effectiveness before releasing it in the market. Hence, compliance with US FDA regulations is mandatory for marketing and distributing medical devices and IVDs in the U.S. Support from US FDA 510(k) consultant can help you easily navigate the FDA’s 510(k) clearance process. We at Pharmadocx Consultants specialize in FDA 510(k) submission.
Best US FDA 510(k) consultant for medical devices and IVDs
The US FDA 510(k) approval process is used to evaluate and authorize the marketing of medical devices and IVDs in the United States. The approval process involves a thorough review of the design, performance, labelling, and manufacturing process of the device. The 510(k) process is primarily applicable to moderate-risk class II medical devices and IVDs. However, a few low-risk class I and high-risk class III devices may also fall under the purview of this regulatory pathway. Notably, US FDA 510(k) is the widely used pathway for medical device and IVD registration in the USA. However, without proper support and guidance, navigating through the complexities of the FDA 510(k) can be tricky.
Pharmadocx Consultants’ expertise and support and can help you easily clear the US FDA 510(k) regulatory process. Multiple FDA 510(k) applicants are forced to abandon their application process, as they fail to steer through it. However, the support of a US FDA 510(k) consultant will be a game changer. Pharmadocx Consultants is a renowned US FDA 510(k) consultant for medical devices and IVDs. Our team of seasoned professionals is well-versed in FDA regulations and submission requirements. We will leverage our expertise to help you achieve the approval and registration in a seamless manner. Additionally, we assist with the registration and listing of devices that are low risk and do not require special controls under the US FDA.
What is US FDA 510(k)?
Depending on the device’s risk level, different pathways for approval are in place. The pathways are 510(k) clearance, premarket approval (PMA), and the de novo classification process. The US FDA 510(k) is used to demonstrate the device in question is as safe and effective as the “substantially equivalent” device being sold legally in the US. This is a premarket submission process. This process involves evaluating and comparing the device with the “substantially equivalent” device already being sold in the US market. This similar product or “substantially equivalent” device is known as a predicate device. Furthermore, 510(k) supporting documents have to be prepared and submitted, as per regulatory requirements. Then, after reviewing the application and verifying the document, the FDA will clear the device for sale and marketing in the US. Additionally, they will issue a 510(k) number. Then, the number with device details will be uploaded to the 510(k) database.
Thus, FDA 510(k) is used to determine whether the medical device/IVD is safe and effective. Then, the device will be legally permitted in the US market. As a US FDA 510(k) consultant, we will help you identify applicable regulations and steer clear through the regulatory pathways.
Major types of FDA 510(k)
- Special FDA 510(k): This type of FDA 510(k) is required when there is a modification in the device but the modification will not impact the intended use of the device.
- Abbreviated FDA 510(k): When the submission is based on FDA guidance documents, demonstrates compliance with special controls for device type, or follows a voluntary consensus standard.
- Traditional 510(k): This is the original 510(k) and can be used in any situation.
FDA 510(k) is applicable for the following:
- All Class I (Non-Exempt) and Class II medical devices
- Domestic medical device and IVD manufacturers who are trying to introduce new devices to the US market will have to secure FDA 510(k)
- Re-labeller’s or Re-packers who make device labelling changes
Looking for US FDA 510(k) consultant for medical device and IVDs?
Fill out the form below & we will get back to you
Medical device and IVD US FDA 510(k) review process
As a seasoned US FDA 510(k) consultant for medical devices and IVDs, we will make this application process a cake walk for you.
- Submission of US FDA 510(k) application: The application is submitted online via the FDA’s eSTAR system.
- Acknowledgement of application receipt: After the application is submitted, the FDA will email an Acknowledgment Letter, provided you have paid the correct user fee and have included a valid eSTAR. Moreover, the letter will include your 510(k) number and the date your application was received. However, if you have not paid the correct user fee or provided an invalid eSTAR, the FDA will send a Hold Letter.
- Submission acceptance review: The FDA authorities will review your application for completeness. If it is incomplete, the FDA will send you an email within 15 days letting you know the same. Then, within 180 days you have to submit the revised application. Once the application is accepted, the FDA will begin the substantive review. The next steps in the application process will be substantive review followed by interactive review or AI request, depending on the situation.
We have listed below the next steps in the application process.
Substantive review
The lead reviewer will carefully examine the 510(k) application. Then, within 60 days of receiving the application, a substantive interaction is conducted with the applicant. It can either be an email confirming that the FDA will proceed with an Interactive Review or the application will move to an Additional Information (AI) request stage. Notably, the AI request will place the application on hold.
Interactive review
In this phase, the lead reviewer gets in touch with the applicant through email or phone calls. If the application moves to the interactive review phase, it may indicate the application will not be held up. Basically, what ever issues are there in the application it will be resolved within the Medical Device User Fee Amendment (MDUFA) timeframe. Moreover, during this phase, the reviewer may request for more information or details. The applicant will be expected to send the requested information to the lead reviewer or FDA.
Additional information
The application will be paused, if the lead reviewer requests AI. Notably, the FDA must receive the complete response to the query within 180 days from the AI request date. If the FDA does not get a complete response within 180 days, the submission will be considered withdrawn. Notably, the response should clearly mention the name, 510(k) number, and date of the FDA’s request.
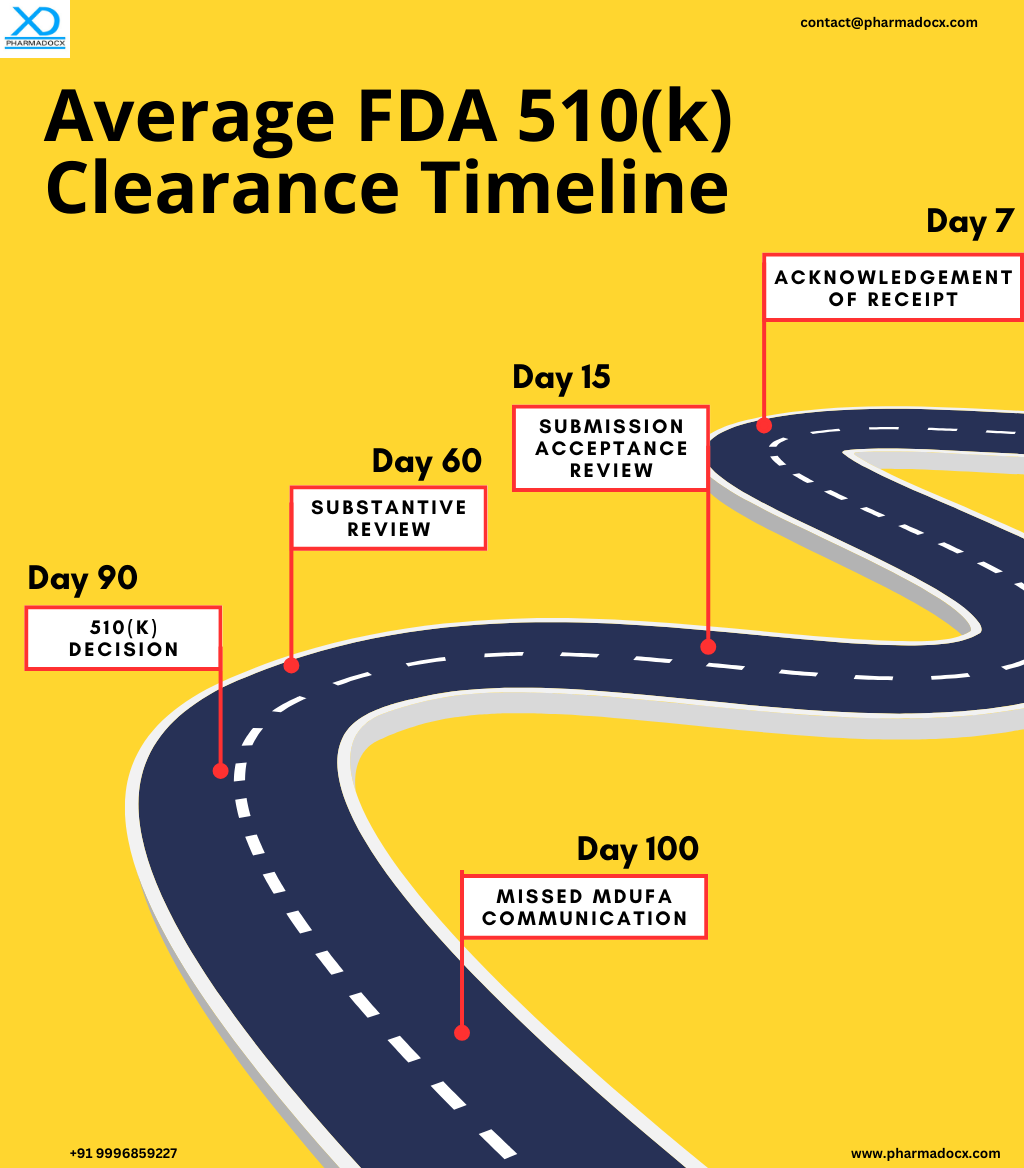
FDA 510(k) decision letter
Within 90 days, the decision is usually made. This excludes any days on hold due to an AI request. The decision can be either “substantially equivalent” (SE) or “not substantially equivalent” (NSE). An SE indicates the 510(k) is cleared. Then, the details will be sent to the applicant via email. However, if the FDA cannot decide within 100 FDA Days, a “Missed MDUFA Communication” is sent. This communication contains the feedback and an estimated completion date.
10 pro tips from the best US FDA 510(k) consultant
As the leading US FDA 510(k) consultant, we have provided some tips that will help you avoid common US FDA 510(k) application mistakes.
- In-depth predicate comparison: It is vital to provide an in-depth comparison with a legally marketed predicate device. The comparison should clearly demonstrate substantial equivalence.
- Compliance with all eSTAR requirements: Your electronic submissions should meet the FDA’s requirements.
- Sufficient testing and data: Sufficient testing should be conducted to collect sufficient data to support the application claims.
- Adequate device description: Clearly describe your medical device or IVD and its intended use. Lack of clarity and details can lead to misunderstandings and delay the application.
- Complete and accurate information: All the required information in the submission should be complete and accurate.
- Pay attention to the pre-submission process: Carefully complete the pre-submission process to address potential concerns before formal submission. Vital issues can be rectified at this stage.
- Identify and address potential risks: Identify and address all potential risks associated with your device. Failing to address them can result in rejection.
- Timely response to FDA requests: Timely response to FDA queries is essential. Delays in communication can prolong the review process, thereby slowdown the application process.
- Proper labelling and instructions: Adequate labelling of the device and clear instructions for use are crucial.
- Compliance with the latest regulatory guidelines: It is vital to stay updated with the latest FDA regulations. Non-compliance with the latest guidelines can lead to submission issues, delays, and even rejection.
Your trusted US FDA 510(k) consultant: Our approach for guaranteed success
Owing to incorrect application, thousands of FDA 510(k) are rejected. Furthermore, in certain cases, the applicants cannot complete their application, as the process is difficult. The Pharmadocx Consultants team is committed to provide proper guidance to their clients to help them successfully secure the approval. We specialize in US FDA 510(k) submission. As a reputed US FDA 510(k) consultant, we follow a unique approach that helps our clients achieve the approval effectively and efficiently. Moreover, we tailor our services as per client requirements.
Our US FDA 510(k) consultant team’s approach
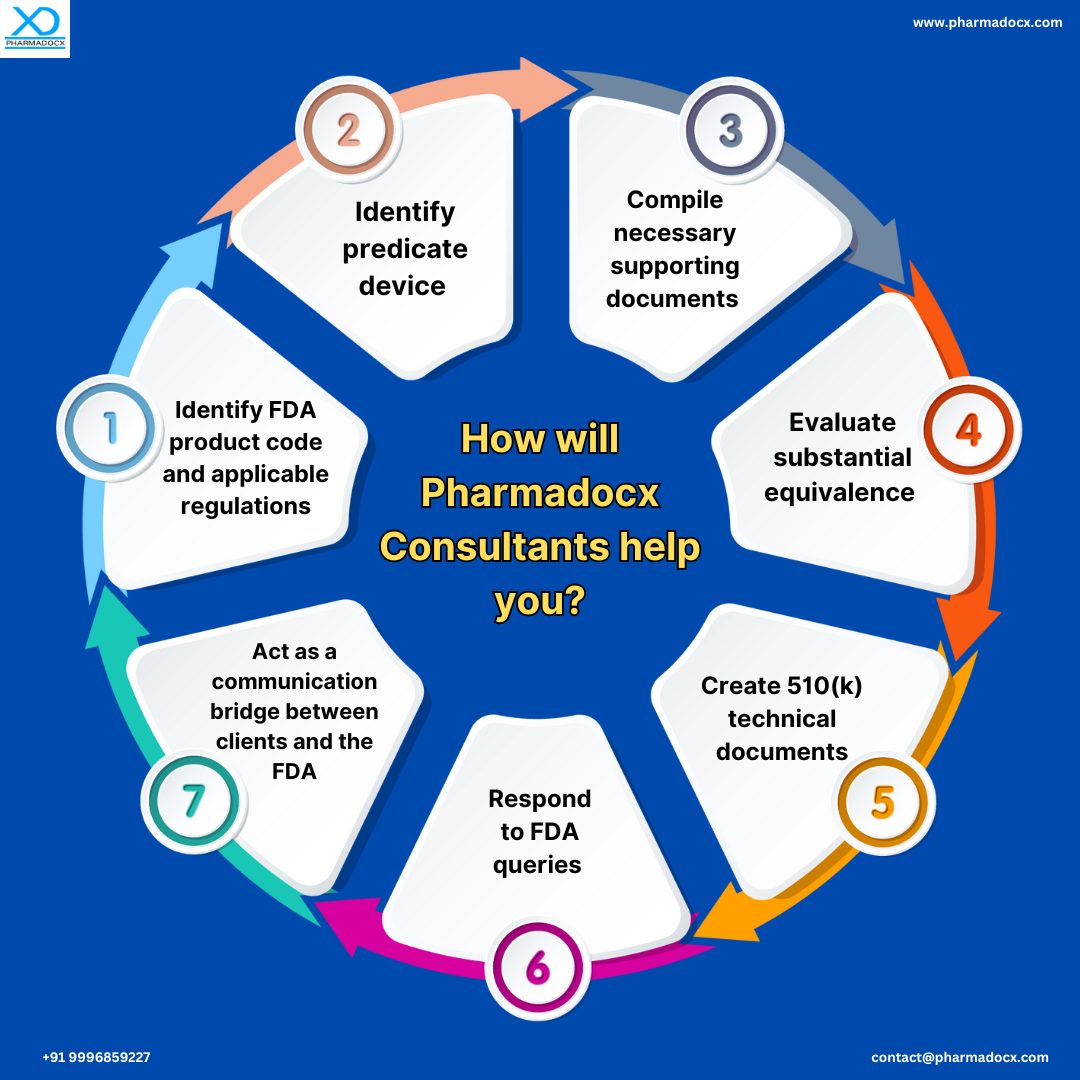
- FDA product code, regulation number, and predicate devices: We double-check the product code selection. This is because the next steps of the application will depend on the product code. Additionally, incorrect code selection can lead to FDA 510(k) application rejection. Furthermore, we careful check whether the appropriate predicate device has been selected.
- Correct identification of testing needs, FDA guidance references, consensus standards, and clinical data requirements: Notably, each product code is associated with particular FDA guidance documents and consensus standards. Our team will thoroughly review the guidelines and zero down on the applicable testing and clinical data requisites. Then, we will guide your team on the necessary testing requirements and potential clinical data that has to be collected.
- Analysis of the required information: We check for any lapses in the required documentation. Our US FDA 510(k) consultant team is seasoned with the knowledge of the required documents. Hence, we utilize our knowledge to find any lapses in your documentation. Moreover, we prepare a report highlighting the information your documents file does not contain but is required for the 510(k) application.
- Smooth FDA 510(k) application: Our US FDA 510(k) consultant team will compile all the necessary documents for the application. Then, we will help you submit the application. Notably, we try to ensure your application is complete with all the information so that it does not move to the AI request stage. However, AI requests do happen often. Thus, we will help you respond to all the queries in a timely manner. Our aim is to help you have a smooth and successful FDA 510(k) application journey.
US FDA 510(k) consultant for Europe-based companies
Are you a Europe-based medical device company planning to launch your products in the US market? Well, you have landed in the right place. We at Pharmadocx Consultants have extensive expertise in the 510(k) application process. The Pharmadocx Consultants team provides support to companies based in Germany, France, UK, Italy, Netherlands, Belgium, Austria, Sweden, Spain, Switzerland, and other European countries. Get in touch with us to simplify your regulatory journey in the US.
As a US FDA 510(k) consultant, how will the Pharmadocx Consultants team help you?
Pharmadocx Consultants has a seasoned team of US FDA 510(k) consultants for medical devices and IVDs. Moreover, we constantly stay abreast of the latest US FDA regulatory guidelines. We will leverage our expertise to help you secure the approval in a hassle-free manner.
We have cleared some FAQS every US FDA 510(k) consultant is asked
When is a device considered identical to the predicate?
A device is considered identical to the predicate only if:
- It has the same intended use as the predicate
- It has the same technological characteristics as the predicate
- Deliberate use is the same as the predicate
- In case the device has different technological characteristics, it should not raise different questions of safety and effectiveness
The data submitted to the FDA should reveal the device is at least as safe and effective as the existing legally marketed device
How do I know whether a medical device has received FDA approval?
FDA’s public database, the 510(k) Premarket Notification Database, provides information on specific devices and their clearance status. You can search this database to check whether a particular medical device has received FDA approval. Alternatively, you can contact the device manufacturer or FDA for confirmation of the device’s FDA approval status.
How long does it take to secure the FDA 510(k) approval?
Usually, the FDA approval process should take 90 days from the date of submission. However, more time may be required owing to various factors, such as the device’s complexity, FDA’s workload, and the completeness of the application.
Related topics
A Guide to Selecting US FDA Agent for Your Company
US FDA Medical Device Registration and Listing
5 Pro Tips for Hassle-free US FDA 510(k) Submission
Differences Between US FDA De Novo and 510(k) Pathway
US FDA De Novo Submission Process and Timeline
Difference Between US FDA 510(k) and PMA
US FDA Regulatory Pathways for New Medical Devices
US FDA 510(k) Dossier Preparation
US FDA 510(k) Third Part Review
Why Does Strategic Timing of US FDA Submission Matter?
US FDA Medical Device Classification System
FDA 510(k) Pathway for Unclassified Devices
Let's Talk!
We'd love to hear from you! Whether you have questions about our pharmaceutical plant setup consultation services or want to discuss a potential project, our team is here to help. Simply fill out the form below, and we'll get back to you as soon as possible. Alternatively, you can reach out to us directly using the phone number or email address listed on this page. We look forward to connecting with you!
Phone / Whatsapp
Address
- Head Office - Opposite Dewan Mill, Old D.C. Road Sonepat - 131001 Haryana, India
- Registered Office - Netaji Subhash Place, Delhi, 110034

