Get CDSCO License for Orthopaedic Medical Devices
Easily get CDSCO License for Orthopaedic Medical Devices in India.
Maximum number of Orthopaedic Plants Setup in India
Fast & hassle-free approvals
Manufacturing & Import Registration
Documentation that passes scrutiny the first time
Turnkey Project Consultation
600+ Granted Approvals 27+ Years Experience
OUR TRUSTED CUSTOMERS












We are one of India’s premier medical device consultants. Our team has extensive knowledge and expertise in CDSCO regulatory guidelines for medical devices. With our support, you can start your orthopaedic medical device business in India in a hassle-free manner. Moreover, the Pharmadocx Consultants team has set up the maximum number of orthopaedic medical device manufacturing facilities in north India. Our team will work relentlessly to ensure you secure the CDSCO license for orthopaedic medical devices in a seamless-manner.
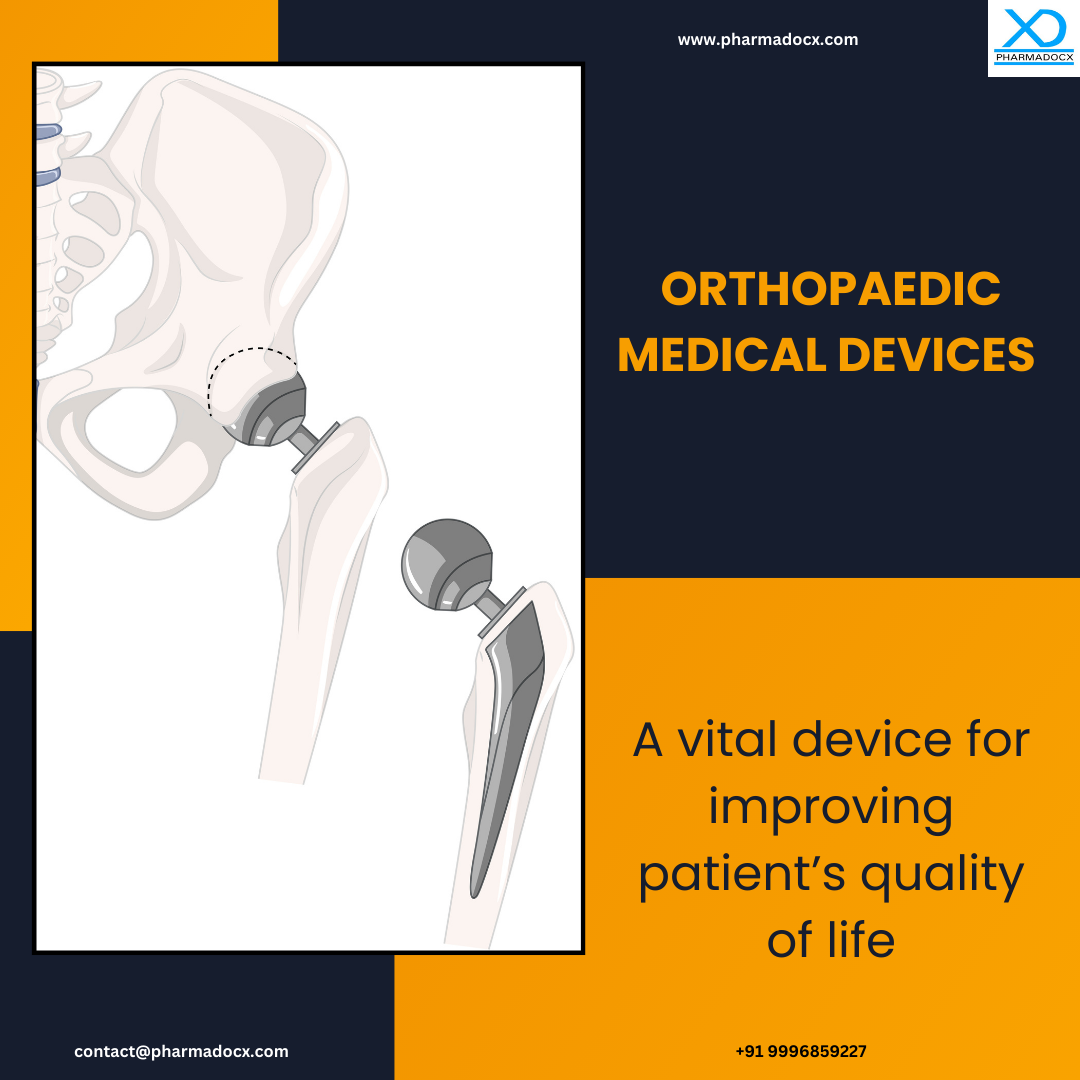
What are Orthopaedic Medical Devices?
Orthopaedic medical devices are a wide range of devices used to stabilize and restore function in bones, joints, and other related structures. These tools and instruments are designed to diagnose, treat, and manage musculoskeletal conditions. Implants, instruments, and orthotics are the major types of orthopaedic medical devices. These orthopaedic medical devices have a vital role in supporting and enhancing an individual’s mobility, thereby improving their quality of life.
Orthopaedic medical devices have a wide range of applications. We have highlighted some of them.
- Orthotics are used to manage chronic conditions, such as arthritis.
- Orthotics and assistive devices can help patients regain function after an injury or surgery.
- Implants and instruments are used to treat fracture.
- Artificial joints are implanted to replace damaged joints.
3 major types of orthopaedic medical devices in hospital settings
- Orthotics: Orthotics are medical devices worn on the body to support or align a limb or joint. Examples: Assistive devices, braces, casts, etc.
- Orthopaedic implants: These implants are used to replace or support damaged or missing parts of the musculoskeletal system.Examples: Spine stabilization implants; fracture fixation implants; artificial hip, knee, or shoulder joints, etc.
- Surgical tools and instruments: These tools and instruments are used by surgeons during orthopaedic surgical procedures. Examples: Bone holding forceps, reamers, drills, saws, retractors, etc.
CDSCO Regulations for Orthopaedic Medical Devices
Central Drugs Standard Control Organization (CDSCO) aims to ensure the safety and efficacy of orthopaedic medical devices in India. Therefore, orthopaedic medical device manufacturers and importers have to be well-versed with the CDSCO regulations for orthopaedic medical devices. Moreover, securing the CDSCO license for orthopaedic medical devices is a mandatory requirement.
CDSCO Classification for Orthopaedic Medical Devices
Medical devices have been classified based on the potential risks associated with their use. Similar to other medical devices, orthopaedic devices have also been classified into the four CDSCO classes, A, B, C, and D. This medical device classification system has been formulated to simplify the CDSCO license application system.
Class A orthopaedic medical devices
- Bone curette
- Bone cutter
- Surgical punch/bone punch
- Surgical Mallet
- Osteotome
- Orthopaedic implant impactor
- Orthopaedic cast padding
- Plaster instruments/sawplaster knife
- Plaster of Paris bandage
- Bone chisel
- Bone tap
- Orthopaedic countersink
Class B orthopaedic medical devices
- Intra osseous fixation wire
- Bone wire
- Bone cap
- Plates, clippers, screws
- Spinal fluid manometer
- Sterilization wrap
- Surgical drill and its attachment
- Ultrasonic cleaner for medical instruments
- Unilateral external fixation system
- Vacuum-powered body fluid suction apparatus
- Hip system instrument
- Bone cement accessories
Class C orthopaedic medical devices
- Orthopaedic implant & accessories
- Pedicle screw spinal system
- Ankle joint metal/composite semi-constrained cemented prosthesis
- Ankle joint metal/polymer non-constrained cemented prosthesis
- Elbow joint humeral (hemi- elbow) metallic uncemented prosthesis
- Finger joint metal/metal constrained uncemented prosthesis
- Spinal intervertebral body fixation orthosis
- Toe joint polymer constrained prosthesis
- Wrist joint carpal lunate polymer prosthesis
- Wrist joint carpal scaphoid polymer prosthesis
Class D orthopedic medical device classes
- Hip joint metal/metal semi-constrained, with a cemented acetabular component, prosthesis
- Hip joint metal constrained cemented or uncemented prosthesis
- Intervertebral body fusion device
- Cervical artificial disc
- Hip joint metal/polymer constrained cemented or uncemented prosthesis
- Hip joint femoral (hemi-hip) metallic resurfacing prosthesis
- Hip joint femoral (hemi-hip) trunnion-bearing metal/polyacetal cemented prosthesis
- A hip joint (hemi- hip) acetabular metal cemented prosthesis
- Hip joint femoral (hemi-hip) metallic cemented or uncemented prosthesis
We have listed some examples of orthopaedic medical devices belonging to the different CDSCO classes. Use our free tool to check which CDSCO class your orthopaedic device belongs to.
CDSCO License for Orthopaedic Medical Devices
CDSCO license for manufacturing class A & B orthopaedic medical devices
MD 5 license is required to manufacture class A & B orthopaedic medical devices. An application has to be filed under Form MD 3. A fee of Rs. 5,000 for the manufacturing license and Rs. 500 for each distinct device is required.
CDSCO loan license for manufacturing class A & B orthopaedic medical devices
Manufacturers seeking to obtain a loan license for manufacturing class A & B orthopaedic medical devices need to apply using the Form MD 4. CDSCO will grant the approval via Form MD 6.
CDSCO license for importing orthopaedic medical devices into India
MD 15 license is required to import orthopaedic medical devices into India. An application has to be filed under Form MD-14.
CDSCO license for manufacturing class C & D orthopaedic medical devices
MD 9 license is required to manufacture class C & D orthopaedic medical devices. An application has to be filed under Form MD-7. A fee of Rs. 50,000 for the manufacturing license and Rs. 1,000 for each distinct device is required.
CDSCO loan license for manufacturing class C & D orthopaedic medical devices
Manufacturers seeking to obtain a loan license for manufacturing class C & D orthopaedic medical devices need to apply using the Form MD 8. CDSCO will grant the approval via Form MD 10.
CDSCO test license to manufacture orthopaedic medical devices in India
MD-13 license is required to manufacture small quantities of devices for testing purposes. An application has to be filed under Form MD-12.
Documents Required for CDSCO License for Orthopaedic Medical Devices
We have enlisted the necessary supporting documents.
- Cover letter
- Organization identity proof
- Sale Deed/Rent Deed of the Premises
- Plant Master File
- Device master file
- Power of attorney
- Building Layout with Dimension
- Documents for the team of qualified and experienced staff who can manufacture and test your medical devices.
- Test License, if required for testing the orthopaedic medical device
- Environmental regulation compliance documents
- Certificate of analysis of 3 consecutive batches
- ISO 13485 Certificate
- TR6 challan
- Quality assurance certificate
Step-by-Step Guide to Applying for CDSCO License for Orthopaedic Medical Devices
- Compile all necessary documents for CDSCO orthopaedic medical device license application.
- Login/signup on the official CDSCO online registration portal, SUGAM portal.
- Fill the appropriate application form.
- Submit the application online along with the necessary supporting documents.
- CDSCO will review the application and verify the documents.
- CDSCO may raise queries that have to be promptly and accurately addressed. The queries can range from clarification of the submitted documents to requests for additional information.
- CDSCO officials may audit the manufacturing facility. Prepare for the CDSCO inspection phase.
- Once all the criteria are satisfied, CDSCO will grant the license.
Need help applying for CDSCO license for orthopaedic medical devices?
Fill out the form below and we will get back to you
Validity of the CDSCO License for Orthopaedic Medical Devices
CDSCO orthopaedic license is valid indefinitely. However, to maintain the validity, the license retention fee has to be paid every 5 years.
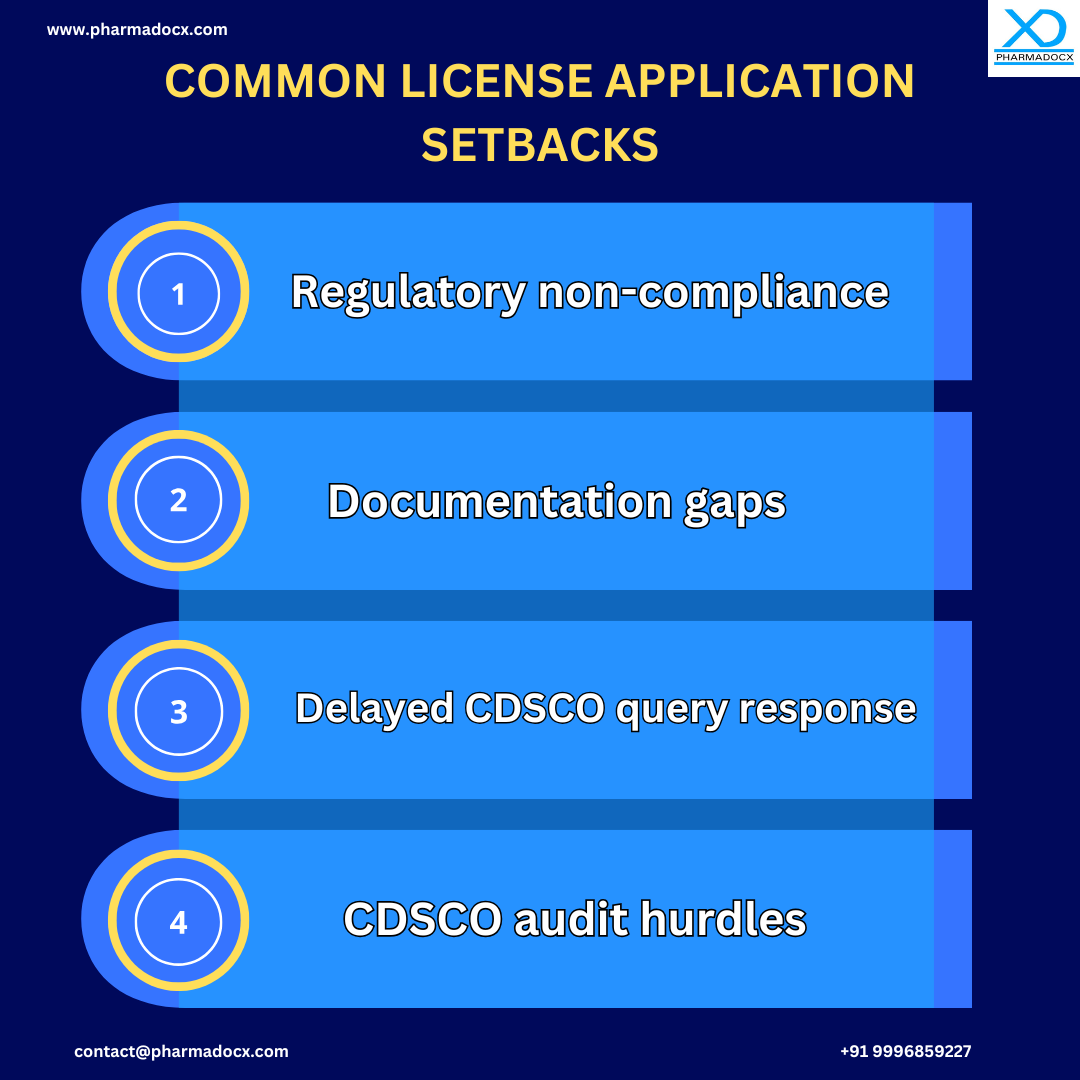
How to Overcome CDSCO License Application Challenges?
- Comply with all CDSCO regulations for orthopaedic medical devices.
- Ensure all documents are up-to-date and meet CDSCO’s guidelines.
- Promptly and accurately address CDSCO queries.
- Conduct regular internal reviews of the facility to avoid any pitfalls during CDSCO regulatory audits.
How can Pharmadocx Consultants Assist in Securing the CDSCO License for Orthopaedic Medical Devices?
- Expert guidance: We have extensive knowledge of the CDSCO regulations. Our team will help you select the appropriate CDSCO license for your product. Additionally, we will help you navigate through the CDSCO regulations for orthopaedic medical devices. Furthermore, we will help you fill out the CDSCO license application form.
- Setting up the manufacturing facility: We have set up the maximum number of orthopaedic medical device manufacturing facilities in north India. We will help you set up your facility per CDSCO regulatory guidelines. With our support, you will not face any regulatory obstacles while securing the CDSCO license.
- Document preparation service: We offer comprehensive document preparation service. Our team will not only help you prepare the necessary documents but also compile them per CDSCO guidelines.
- Query resolution support: In case CDSCO raises any query regarding the license application, we will help you promptly respond to it.
- End-to end service: Our support does not end with license application. We will provide support till you successfully secure the CDSCO orthopaedic medical device license. Moreover, we provide continued support for compliance with any updates in the CDSCO regulations.
Why choose Pharmadocx Consultants?
Medical Device Licences
Years Experience
Plants Set-up
Our Clients

Want to easily secure the CDSCO license for orthopaedic medical devices? Call/Whatsapp on 9996859227 or email at [email protected] to let us help you turn your medical device business dream into a licensed reality.
Let's Talk!
We'd love to hear from you! Whether you have questions about our pharmaceutical plant setup consultation services or want to discuss a potential project, our team is here to help. Simply fill out the form below, and we'll get back to you as soon as possible. Alternatively, you can reach out to us directly using the phone number or email address listed on this page. We look forward to connecting with you!
Phone / Whatsapp
Address
- Head Office - Opposite Dewan Mill, Old D.C. Road Sonepat - 131001 Haryana, India
- Registered Office - Netaji Subhash Place, Delhi, 110034


