CDSCO MD 13 Test License For Manufacturing Medical Devices
Easily get CDSCO MD13 License in the shortest time with least regulatory hurdles.
For manufacturing medical devices for testing purposes, CDSCO MD13 License is required. This is granted by Central authorities.
Expert consultation with industry leaders
Fast & hassle-free MD13 approval
Manufacturing Registration
Documentation that passes scrutiny the first time
Fast and accurate query replies
600+ Granted Approvals 27+ Years Experience
300+ Happy Clients












Medical devices have a significant impact on patient safety and outcome. They form the backbone of the healthcare industry, as they are used to diagnose, monitor, and treat patients. In India, the demand for medical devices is high. However, India is still heavily import dependant for medical devices. New entities are planning to venture into the medical device manufacturing industry to cater to the demands. The Indian regulatory guidelines require the medical devices entering the Indian market be tested for quality, safety, and efficacy. These guidelines are in place to ensure substandard unsafe medical devices do not enter the Indian market. Thus, Indian regulatory guidelines control the medical device testing process in India. CDSCO MD 13 test license is required to manufacture medical devices for testing purposes.
Indian regulatory guidelines require all medical devices be tested for quality, safety, and efficacy. Moreover, the Indian regulations do not permit the manufacture of medical devices even for testing purposes without proper licenses. A CDSCO medical device test license (MD 13) is mandatorily required to test devices. The CDSCO test license ensures the medical devices is manufactured solely for testing and not for commercial purpose. By issuing this license, CDSCO ensures the devices are properly tested and assessed before they are marketed in India. The license is a vital component of Indian medical device regulatory framework. Only after proper clinical evaluation and testing, the company may apply for permanent CDSCO manufacturing license.
What is CDSCO MD 13 Test License?
CDSCO MD 13 test license permits manufacturing small quantities of medical devices for testing purpose only and not for sale. The MD 13 license permits manufacturers to produce Class A, B, C, and D medical devices for the following purposes:
- Testing and evaluation: The license is used to test and evaluate the medical device’s performance, safety, and reliability.
- Clinical investigation: Conducting clinical trials using the medical device to collect data on safety and efficacy.
- Demonstration: Demonstrating the intended use and quality of the medical device to potential stakeholders, such as healthcare professionals or investors.
- Training: Providing training on how to use or operate the medical device.
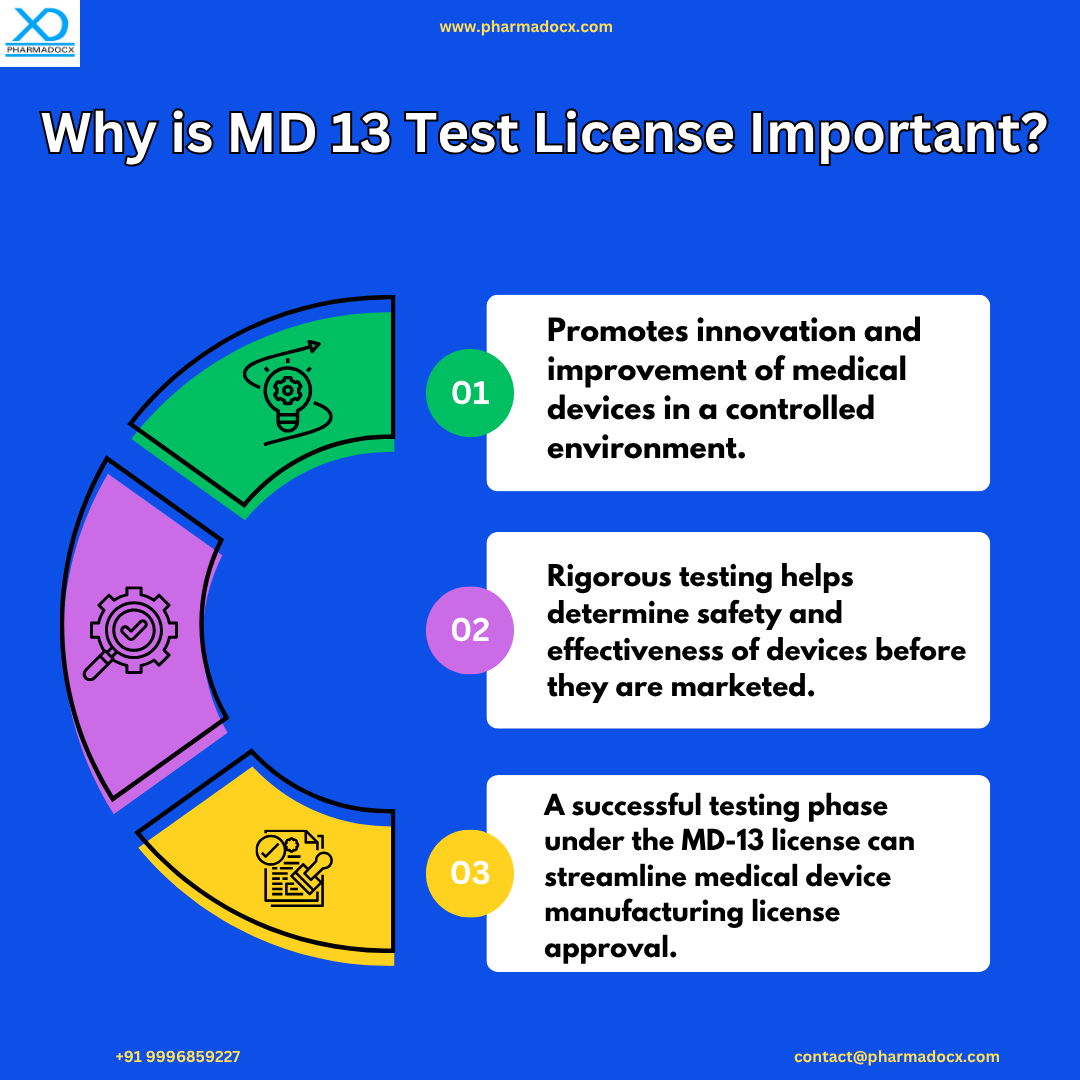
The MD-13 license is a valuable resource for manufacturers to develop and assess medical devices in controlled settings. This license aims to promote innovation, quality assurance, and advancements in the medical device manufacturing industry without the need for a manufacturing license. The CDSCO license allows manufacturers to collect data on device safety and efficacy. Additionally, it allows manufacturers to obtain feedback on the performance of their devices and to demonstrate the safety and efficacy of their devices to regulatory authorities. The data and feedback obtained by manufacturing medical devices under the test license can be used to improve medical devices, thereby revolutionise patient care in India.
MD 12
For the CDSCO medical device test license, the manufacturer needs to apply using the Form MD-12.
MD 13
CDSCO will grant the test license for manufacturing medical devices for testing purposes under Form MD-13.
The CDSCO MD 13 test license allows medical device manufacturers to conduct essential trials and evaluations of medical devices in compliance with established regulations.
3 Major CDSCO Test License MD-13 Obligations
- License usage criteria: The licensee is permitted to use the license exclusively for tests, evaluations, clinical investigations, examinations, demonstrations, and training at the place specified in the license.
- Recordkeeping: The licensee has to maintain a record of the quantity of medical devices manufactured, tested, and stocked and disposed.
- Manufacturing premise access: The licensee is required to allow the medical device officer to enter the premises with or without notice. The officer will check whether only authorized activities, such as tests, evaluations, clinical investigations, demonstrations, or training, are being performed.
Licensing Authority
Central Drugs Standard Control Organization (CDSCO) grants the MD 13 test license.
Timeline for Approval of MD 13 License Application
Timeline for securing the CDSCO MD 13 license is approximately 1.5 to 2 months.
Fees Required for MD 13 License Application
The fee for MD 13 license application is Rs 500 per product.
Who can Apply for CDSCO MD 13 License?
Any entity who wishes to manufacture Class A, B, C, or D medical devices for testing purpose can apply for CDSCO MD 13 license.
Documents Required to Get CDSCO MD 13 Test License
- Covering letter
- Medical device details, including intended use, the material of construction, specifications, and design.
- Undertaking stating the medical device will be used for testing and not for commercial purposes.
- A statement justifying the proposed quantity of medical devices to be manufactured for testing purposes.
- List of equipment and instruments
- List of qualified personnel
- For medical devices produced for clinical investigation, a detailed testing plan is required. The plan should present the specific tests, number of patients involved, and study duration, etc.
- The place where the demonstration, evaluation, testing, clinical trials, and training will be performed should be specified.
- Quality Management Certificates, such as ISO 13485, ISO 9001.
- Proper medical device labelling and instructions for use (IFU), including any warnings or precautions, should be clearly provided.
- An undertaking from the laboratory where the tests will be performed stating the availability of the required facilities.
- Schematic plan of premises
- Fees receipt
- Approval letter authorizing research and development activities issued by any government organization, if any.
Need help applying for MD 13 Test License?
Contact us now
CDSCO MD 13 Test License Application: Step-by-step Guide
- Register on SUGAM: Create an account on the SUGAM portal and register your company. Login to the application portal.
- Fill in the application: Fill in the application Form MD-12 for CDSCO test license for manufacturing medical devices.
- Upload documents: Upload all the necessary supporting documents per CDSCO MD 13 document checklist.
- Pay the fees: Pay the applicable license application fees
- Submit the application: Submit the application on the online portal.
- Application review: CDSCO will review the application and verify the documents. They will evaluate the authenticity and completeness of the submitted documents. Additionally, they will check the application for eligibility for the MD-13 license application grant.
- License grant: Once all the criteria are satisfied, CDSCO will grant the license. The regulatory body will issue the medical device test license under MD-13 for manufacturing medical devices for testing purpose.

Pro tips for CDSCO MD 13 Test License
- Maximum 10 items are allowed per application.
- The test protocol is a crucial requirement for the MD 13 license application.
- The quantity of devices to be manufactured must be justified during the application.
- The specifications of the medical device, such as intended use, material, and description, must be provided.
- Batch details, quantity to be used, and quantity to be retained have to be mentioned.
- Test licenses must be used exclusively for the purpose for which they were secured.
Pharmadocx Consultants: Your Trusted Ally for Easily Securing the CDSCO MD 13 Test License
CDSCO MD 13 test license helps medical device manufacturers test their products before launching them. Having various documentation needs and application requirements, the MD 13 test license application process is cumbersome and time consuming. With the support of our team of experts, you can secure the license in a breeze. Email at [email protected] or call/Whatsapp on 9996859227 and our experts will help you navigate the process. We will ensure you obtain your CDSCO medical device test license without major hiccups. Our expertise in document preparation, license application, and regulatory guidelines will make the process seamless.
Let's Talk!
We'd love to hear from you! Whether you have questions about our pharmaceutical plant setup consultation services or want to discuss a potential project, our team is here to help. Simply fill out the form below, and we'll get back to you as soon as possible. Alternatively, you can reach out to us directly using the phone number or email address listed on this page. We look forward to connecting with you!
Phone / Whatsapp
Address
- Head Office - Opposite Dewan Mill, Old D.C. Road Sonepat - 131001 Haryana, India
- Registered Office - Netaji Subhash Place, Delhi, 110034

