Best Consultant for Revised Schedule M Implementation in India
The Indian government is aiming to bring the Indian pharmaceutical regulations at par with international standards. Hence, drugs manufactured in India would be of international standard and be globally accepted. Thus, the Schedule M guidelines have been revised to update the regulatory requirements for manufacturing pharmaceuticals in India. Therefore, manufacturers have to mandatorily ensure strict compliance with Revised Schedule M guidelines. However, ensuring revised Schedule M compliance is not an easy task. With over 27 years of experience, the Pharmadocx Consultants team has extensive knowledge of the Revised Schedule M guidelines. As a consultant for Revised Schedule M, our support can make this cumbersome task easy for you.
Pharmadocx Consultants for hassle-free Revised Schedule M compliance
Schedule M is being mandatorily implemented in India. Schedule M is a part of Drugs and Cosmetics Act 1940. It specifies the Good Manufacturing Practices (GMP) for pharmaceuticals. GMP, a regulatory benchmark, controls all aspects of the drug-production process for improved patient safety and outcome. Thus, all pharmaceutical manufacturing units in India need to strictly comply with the Schedule M guidelines. Notably, the focus of Schedule M is the production of highest quality pharmaceuticals in India. For further increasing the accountability of pharmaceutical manufacturers, the Indian pharmaceutical regulatory body has revised and updated the Schedule M. Guidelines for premises, plant, and equipment for manufacturing pharmaceutical products have been introduced in addition to GMP guidelines. Thus, you have to ensure compliance with the additional requirements introduced in the Revised Schedule M guidelines. Hence, a consultant for Revised Schedule M implementation can streamline and simplify this process for you.
With extensive experience and expertise in this domain, we understand the challenges commonly faced while achieving these new standards. As India’s premier consultant for Revised Schedule M, we will ensure your pharma company effectively complies with the new regulations in a timely manner. With our guidance and support, pharmaceutical manufacturers can achieve Revised Schedule M compliance in a hassle-free manner.
What is the Revised Schedule M?
First, we need to understand why did the Indian pharma regulatory body aim to revise the Schedule M. New frontiers, such as advanced therapies, personalised medicine, gene and stem cell therapies, have emerged in the healthcare sector. Thus, the pharmaceutical manufacturing processes have been rapidly evolving. With the rapid changes in the pharmaceutical domain, new and updated regulatory guidelines were required. These have fuelled the need for revision in the existing Indian pharmaceutical manufacturing regulatory guidelines. The principles and concepts of the existing Schedule M regulatory guidelines had to be changed. The government aimed to revise the Schedule M to bring the Indian pharmaceutical regulations at par with global standards. Thus, drugs manufactured in India would be globally accepted. This will fulfil the vision of establishing India as a global pharma manufacturing hub.
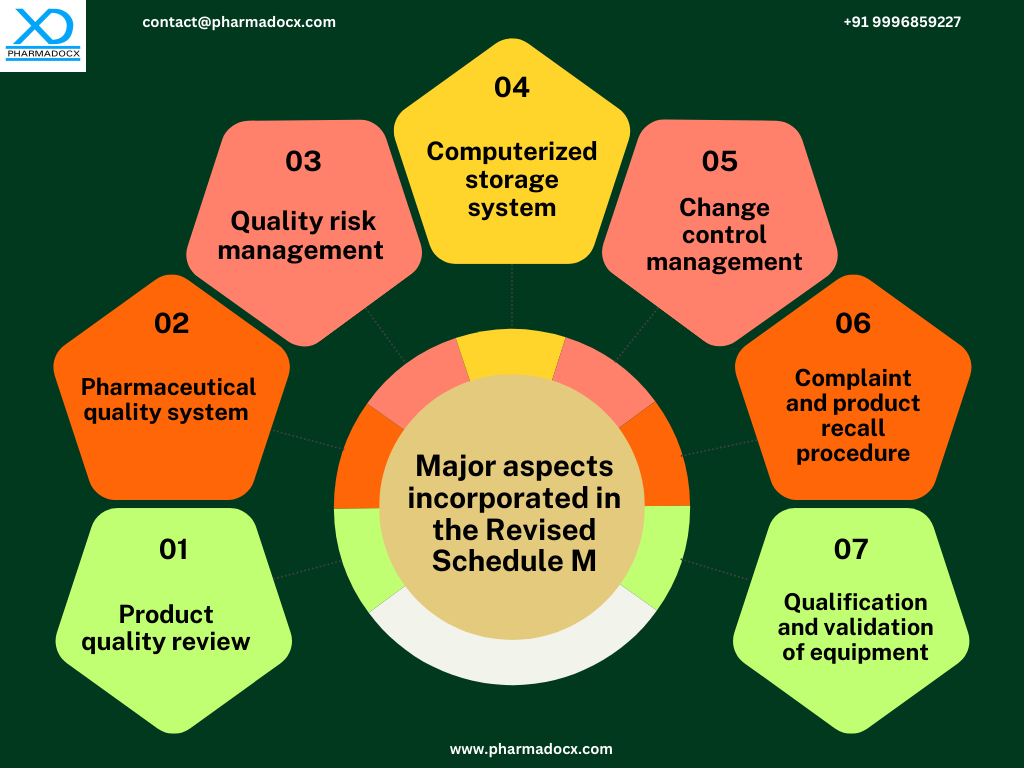
Significant changes have been made in the Schedule M guidelines to safeguard patients and improve the Indian pharmaceutical industry’s reputation. Notably, the changes mainly include incorporation of additional requirements in addition to the existing GMP guidelines. The Revised Schedule M has placed special focus on risk management, qualification and validation of equipment. Guidelines for premises, plant, and equipment for manufacturing pharmaceutical products have been introduced along with GMP guidelines in the revised Schedule M. Additionally, the provision for self-inspection has been increased. Thus, by effectively implementing Revised Schedule M guidelines, high-quality, effective, and safe drugs will be manufactured in India.
Pharmaceutical quality systems and product quality review have been placed at the core of the revised Schedule M. Furthermore, the manufacturers are required to market the drugs only after obtaining satisfactory quality test results. Additionally, a small quantity of sample drugs from a batch should be stored in case the tests have to be repeated for quality verification.
Consultant for Revised Schedule M: How can Pharmadocx Consultants help you seamlessly implement revised Schedule M guidelines?
Excellence in pharmaceutical product quality and enhanced patient safety will be possible with the strict enforcement of Revised Schedule M. Hence, the Indian pharma regulatory body is mandatorily enforcing the Revised Schedule M. However, the Revised Schedule M has various specifications and requirements. Extensive knowledge of the guidelines will be required to properly implement the guidelines. Thus, taking the help of a consultant for Revised Schedule M implementation, will be beneficial.
The Pharmadocx Consultants team has extensive knowledge of the Revised Schedule M guidelines, regulations, and requirements. We are India’s premier consultant for Revised Schedule M implementation and compliance in pharma companies. Our team of experts will provide holistic support to help you avoid any regulatory sanctions. We will leverage our rich experience and in-depth expertise in this field to help you. With our support, you can rest assured compliance with all the clauses of the revised Schedule M guidelines.
Looking for a consultant for Revised Schedule M implementation in your pharma company?
Fill the form below and we will get back to you
Support provided as a consultant for Revised Schedule M implementation in pharma company
- Regulatory compliance: We will help you ensure that your manufacturing facility meets Revised Schedule M and WHO-GMP regulatory standards. Moreover, we guarantee compliance with all the clauses of Schedule M. Additionally, we will provide a detailed checklist for your easy reference.
- Document preparation support: Our team will help you prepare all the documents required for compliance with Schedule M.
- Modification of plant: We will help you modify your manufacturing plant to comply with the new Schedule M guidelines and specifications.
- Staff training: As a consultant for Revised Schedule M, we will train your staff to equip them with the necessary knowledge of the revised guidelines. Our comprehensive training will make your technical staff adept at handling manufacturing facility inspections and audits.
- Mock audit: Our team will conduct thorough mock audits at your manufacturing facility. We not only identify the shortcomings but also provide corrective actionable next steps. We will work relentlessly to make your manufacturing facility audit ready.
Revised Schedule M guidelines also apply to existing pharma companies
Existing pharma companies will also have to comply with the revised Schedule M guidelines. We will help renovate your existing pharma factory as per the revised guidelines. Our team of experts will completely modify your factory within the deadline so that you do not face legal consequences. We will revamp your factory as per updated guidelines at minimal expenditure with minimum operational disruption within the stipulated deadline.
Why choose Pharmadocx Consultants?
Drugs Licences
Years Experience
Plants Set-up
Our Clients

Why should pharma companies in India bother with the Revised Schedule M?
- Compliance with Revised Schedule M guidelines is now a mandatory requirement for manufacturing pharmaceuticals in India. Non-compliance can attract fines and penalties as well as regulatory sanctions.
- Compliance with Revised Schedule M would indicate high-quality, safe, and effective drugs are being manufactured.
- Will demonstrate commitment to enhanced patient outcome and safety.
- Pharma companies will aim to continuously improve their pharmaceutical manufacturing process to deliver high quality products.
- Companies will have to adopt efficient quality management systems.
- Will indicate compliance with most global pharmaceutical regulatory standards.
Consultant for Revised Schedule M: Why choose Pharmadocx Consultants?
- Extensive expertise in Revised Schedule M guidelines and CDSCO regulations
- Client-centric approach and tailored services to meet specific client needs
- Streamlined, thorough, and time efficient process
- Comprehensive and holistic support for pharma companies
Let's Talk!
We'd love to hear from you! Whether you have questions about our pharmaceutical plant setup consultation services or want to discuss a potential project, our team is here to help. Simply fill out the form below, and we'll get back to you as soon as possible. Alternatively, you can reach out to us directly using the phone number or email address listed on this page. We look forward to connecting with you!
Phone / Whatsapp
Address
- Head Office - Opposite Dewan Mill, Old D.C. Road Sonepat - 131001 Haryana, India
- Registered Office - Netaji Subhash Place, Delhi, 110034