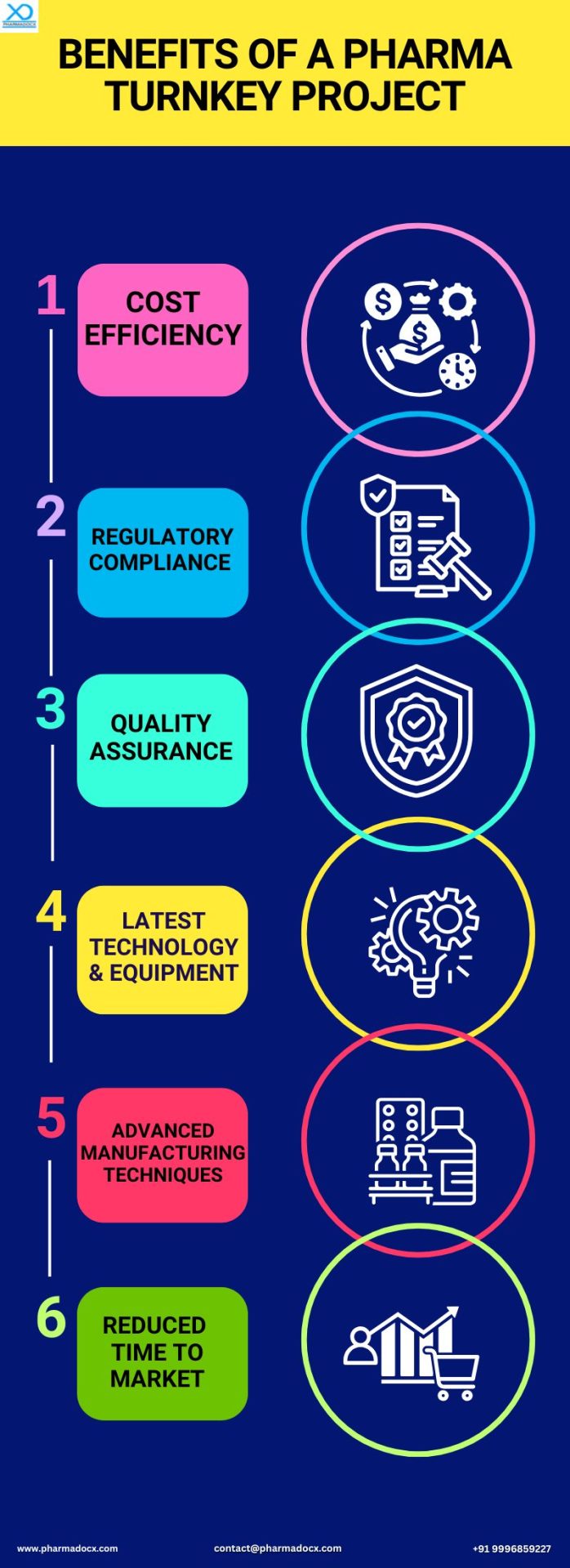
Best Pharma Turnkey Project Service for Pharma Companies
We at Pharmadocx Consultants provide comprehensive start-to-finish (turnkey) solutions for pharmaceutical companies. We deliver a fully functional pharmaceutical facility ready for immediate use. It is the perfect solution for clients seeking efficiency, predictability, operational excellence, and strict regulatory compliance. We are one of the leading pharma turnkey project consultants in India. By availing our pharma turnkey project service, you can significantly cut down on time and resources required for setting up a pharmaceutical manufacturing facility. Moreover, having established more than 500 state-of-the-art pharmaceutical facilities in India, we offer exceptional services to our client. We are committed to building long-lasting partnerships with our clients.
What is a Pharma Turnkey Project Service?
A pharma turnkey project service refers to a comprehensive solution that will deliver a fully operational pharmaceutical production unit ready to turn the key. The pharmaceutical factory will be ready for the company to begin manufacturing their products. The pharma turnkey project consultant will handle every aspect of setting up a new pharmaceutical manufacturing facility. They will take care of the project from initial planning, factory design, construction to equipment installation, validation, and regulatory compliance.
4 key aspects of pharmaceutical turnkey project service
- Complete project management: The pharma turnkey project consultant oversees every stage of the project. The service covers site selection, feasibility studies, design, engineering, construction, equipment installation, validation, and regulatory support. Basically, it covers everything from planning to project completion.
- Expertise in pharmaceutical industry: The pharma turnkey project consultants will have extensive expertise in pharmaceutical industry. They will have in-depth knowledge of pharmaceutical processes, equipment, and regulatory requirements. Hence, they will be able to provide guidance and support to new companies venturing into the pharmaceutical sector.
- Compliance with regulatory guidelines: Pharmaceutical turnkey project services will ensure compliance with pharmaceutical industry regulations, such as GMP, cGMP, WHO-GMP. Additionally, adherence to CDSCO pharma regulations is a must.
- Tailored solutions: A pharma turnkey project service is usually tailored to suite client needs. The project is customized depending on dosage forms (tablets, capsules, and injectables), specific manufacturing processes, and target production capacity.
What is covered under the scope of a pharma turnkey project?
- Site design and planning: Developing the overall pharmaceutical manufacturing facility project layout. The planning stage should focus on production flow, regulatory compliance, and operational requirements.
- Detailed engineering drawings: Creating detailed pharma plant layout drawings and specifications for equipment and the facility.
- Equipment procurement and installation: Sourcing and installing necessary equipment and machinery for manufacturing the pharma product.
- Cleanroom construction: Designing and constructing cleanrooms according to pharmaceutical industry requirements.
- Validation services: Performing required validation activities to ensure process reliability.
- Training: Providing necessary training to manufacturing facility operators and commissioning the production lines.
- Regulatory compliance: Ensuring regulatory compliance at all stages for consistent product quality.
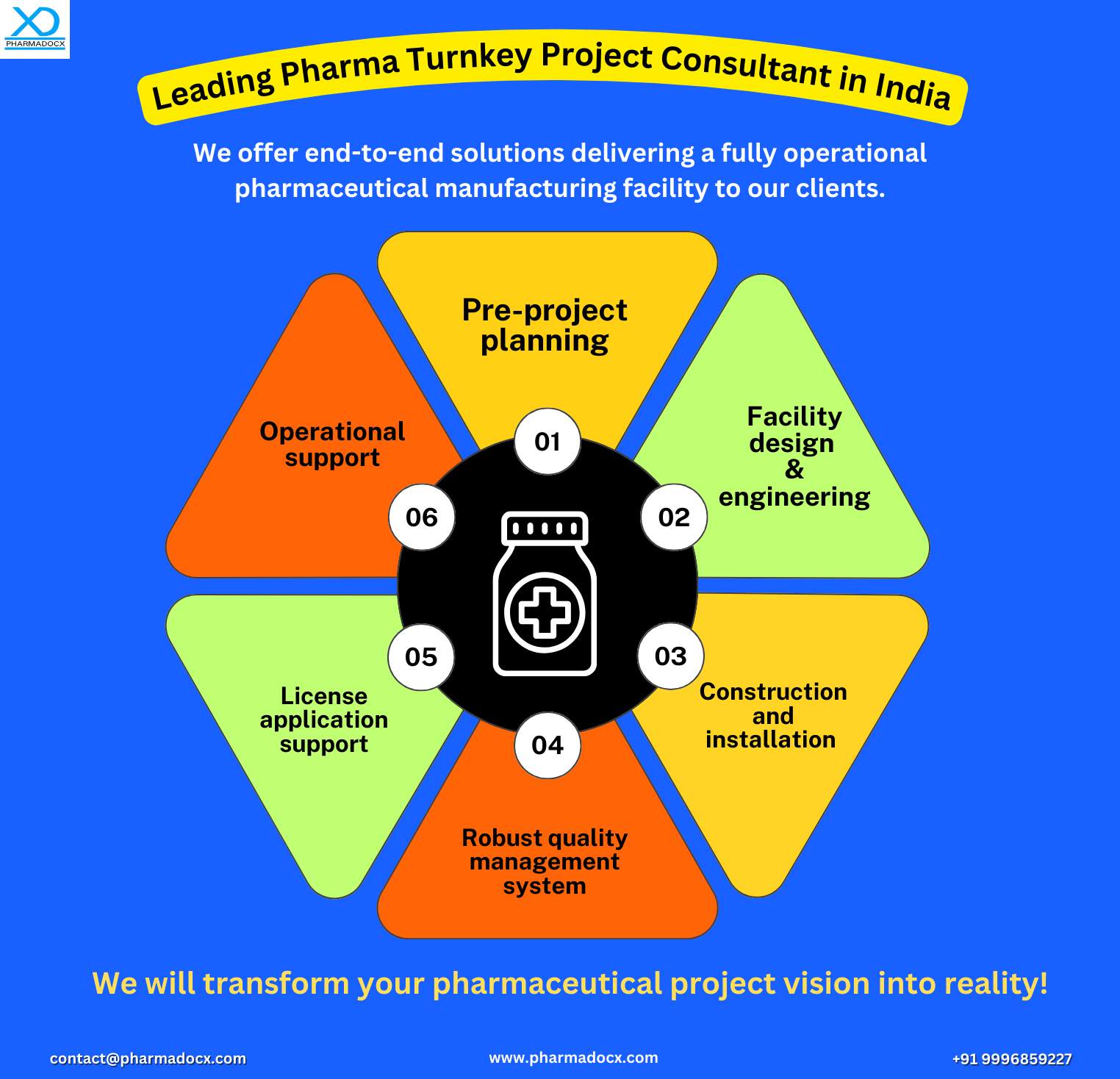
Pharma Turnkey Project Service: Transitioning Your Pharmaceutical Project Vision into Reality
Step 1: Pre-project phase: Planning & feasibility study
- Client requirement analysis: The first step of pharma turnkey project service is to understand the specific requirements of the client. The project planning has to be done based on the intended product type (e.g., tablets, injectables), production capacity, and other specific client requirements.
- Market research and feasibility study: Analysing the target market potential and competition. The technical, economic, and regulatory feasibility of the project are evaluated to ensure its viability.
- Business plan development: Based on the feasibility study findings, a comprehensive business plan detailing goals, strategies, and financial projections has to be developed.
- Site selection: Finding a suitable location for establishing the pharmaceutical plant is vital. Factors, such as local regulations, logistics, environmental impact, and transport connectivity, are important parameters.
Step 2: Facility design and engineering
- Facility design: Creating detailed plant layout designs for the pharma manufacturing facility. Additionally, the facility should comply with GMP, GLP, and other relevant industry standards. Furthermore, focus should be on production capacity, operational requirements, and smooth movement of material and personnel. Moreover, the layout of the production lines, including workflow for manufacturing, quality control, and packaging, has to be carefully designed.
- Machinery selection and procurement: Identifying and procuring the necessary machinery and equipment for manufacturing the pharma product.
- Civil and structural engineering: Overseeing the construction of the pharmaceutical plant per plan and industry standards.
- Cleanroom design: Cleanrooms should be designed with stringent sterility and contamination control standards in mind.
- Infrastructure and utility design: Water supply, power supply, HVAC systems, and other infrastructural requirements have to be focussed on.
Step 3: Construction and installation
- Construction management: Managing the pharma facility construction and ensuring compliance with the approved designs. This covers civil, structural, and mechanical work. Additionally, utilities, such as HVAC, water, and electrical systems, are taken care of.
- Equipment and machinery installation: Installing pharmaceutical manufacturing equipment and machinery.
- Quality control and assurance: Ensuring the entire pharma facility abides by quality standards and GMP guidelines.
- Validation, qualification, and commissioning: Checking whether all systems and equipment are installed correctly and functioning according to design specifications. Additionally, conducting process and equipment validation to ensure proper compliance.
Step 4: Regulatory compliance and license procurement
- Compliance with GMP and regulatory guidelines: Ensuring that the pharma manufacturing facility is designed and constructed as per regulatory guidelines. Compliance with GMP, cGMP, and WHO-GMP is a must.
- Documentation: Preparation of the necessary supporting documents is required for regulatory approvals. The documents have to be prepared and compiled per regulatory requirements.
- Staff training: Conducting workshops to train the client’s staff on regulatory compliance, safety standards, and industry operating procedures. Additionally, preparing the personnel for regulatory audits.
- License application: Helping the client navigate through the complex regulatory guidelines. Additionally, providing the necessary drug manufacturing license application support and assistance to the client.

Step 5: Technology transfer
- Formulation and process transfer: Assisting in the smooth transition of the client’s product formulation and manufacturing processes from the laboratory or pilot scale to commercial-scale production.
- Setting up and calibrating the equipment: Proper calibration of all the equipment. Ensuring that they meet production requirements of the facility.
- Standard operating procedures: Developing standard operating procedures (SOPs) for all stages of production, from raw material procurement to final packaging. Furthermore, ensuring proper implementation of all the SOPs.
Step 6: Operational support
- Production planning and scheduling: Planning the pharma production process. Additionally, optimizing the production processes and scheduling as required.
- Assisting with initial starting of the facility: Providing ample support and guidance for starting the facility to avoid bottle necks and hurdles. Helping the client with the smooth running of the facility and ramping up of the facility to full production capacity.
- Maintenance support: Offering maintenance service and troubleshooting support to the client to ensure the facility operates without disruptions.
- Logistics: Helping the client procure raw materials. Providing support for handling storage and transportation of finished pharmaceutical products.
- Regulatory compliance: Ensuring ongoing regulatory compliance during drug manufacturing.
Step 7: Quality assurance and control systems
- Implementation of QMS: Implementing a robust quality management system (QMS) to ensure the standard of product quality is consistently maintained.
- Analytical quality laboratories: Setting up quality laboratories for raw material, in-process quality, and finished product testing.
- Automation of systems: Incorporating latest technologies to automate systems in order to streamline operations, reduce human error, and ensure traceability.
Step 8: Handover & continued support
- Handover of facility to client: The final stage of pharma turnkey project service is handing over the fully functional manufacturing facility to the client. Additionally, the client is provided all necessary documentation and training required to operate the facility. Moreover, the client is provided validation reports and operational guidelines.
- Environmental impact assessment: Conducting environmental impact assessments and ensuring compliance of the facility with all applicable standards.
- Document and record management: Establishing and maintaining comprehensive record maintenance systems.
- Warranty and continued support: Warranty is usually offered for both equipment and the facility itself. Additionally, long-term support is provided to the client in order to ensure the facility continues to efficiently operate.
Why Choose our Pharma Turnkey Project Service?
Having over 27 years of experience, we have served more than 600 clients. Our firm has helped set-up the maximum number of plants in north India.
- In-depth market research
- Robust business plan
- Appropriate site selection
- Regulatory support
- Hassle-free license procurement
- Efficient facility construction and equipment installation
- Expert project management
- Timely completion of the project
- State-of-the-art manufacturing facility
- Adherence to the highest quality standard
- Operational excellence
Looking for pharmaceutical turnkey project solutions?
Fill out the form below to receive expert support
Our clients

Why choose Pharmadocx Consultants?
Drugs Licences
Years Experience
Plants Set-up
Best Pharma Turnkey Project Service in India
We understand the difficulties of establishing a successful pharmaceutical business in India. Hence, we offer a comprehensive suite of cost-effective tailored pharma turnkey project service to our clients. Email at [email protected] or call/Whatsapp on 9996859227 to avail our all-encompassing pharmaceutical turnkey project service.
Let's Talk!
We'd love to hear from you! Whether you have questions about our pharmaceutical plant setup consultation services or want to discuss a potential project, our team is here to help. Simply fill out the form below, and we'll get back to you as soon as possible. Alternatively, you can reach out to us directly using the phone number or email address listed on this page. We look forward to connecting with you!
Phone / Whatsapp
Address
- Head Office - Opposite Dewan Mill, Old D.C. Road Sonepat - 131001 Haryana, India
- Registered Office - Netaji Subhash Place, Delhi, 110034
