CDSCO MD 17 Test License to Import Medical Devices
Easily get CDSCO MD 17 License in the shortest time with least hurdles.
CDSCO MD 17 License is required to import medical devices for testing purposes. This is granted by Central authorities.
Expert consultation with industry leaders
Fast & hassle-free MD17 approval
Import Registration
Documentation that passes scrutiny the first time
Fast and accurate query replies
600+ Granted Approvals 27+ Years Experience
300+ Happy Clients












CDSCO MD 17 test license is required to import medical devices into India for testing, evaluation, demonstration, or training purposes. The MD 17 license application process is complex. It requires an understanding of the regulatory guidelines, documentation needs, license obligations, and post-license responsibility. The Pharmadocx Consultants team will streamline your application. We are market leaders in medical device regulatory consultancy, with a deep understanding of the CDSCO guidelines. Our seasoned experts will provide end-to-end assistance throughout the MD 17 license application process. Additionally, we have a proven track record of successful MD 17 license application. Moreover, our consultants will tailor their approach to your specific product, market, and business goals. By choosing Pharmadocx Consultants, you will be partnering with a team dedicated to your success in the medical device market.
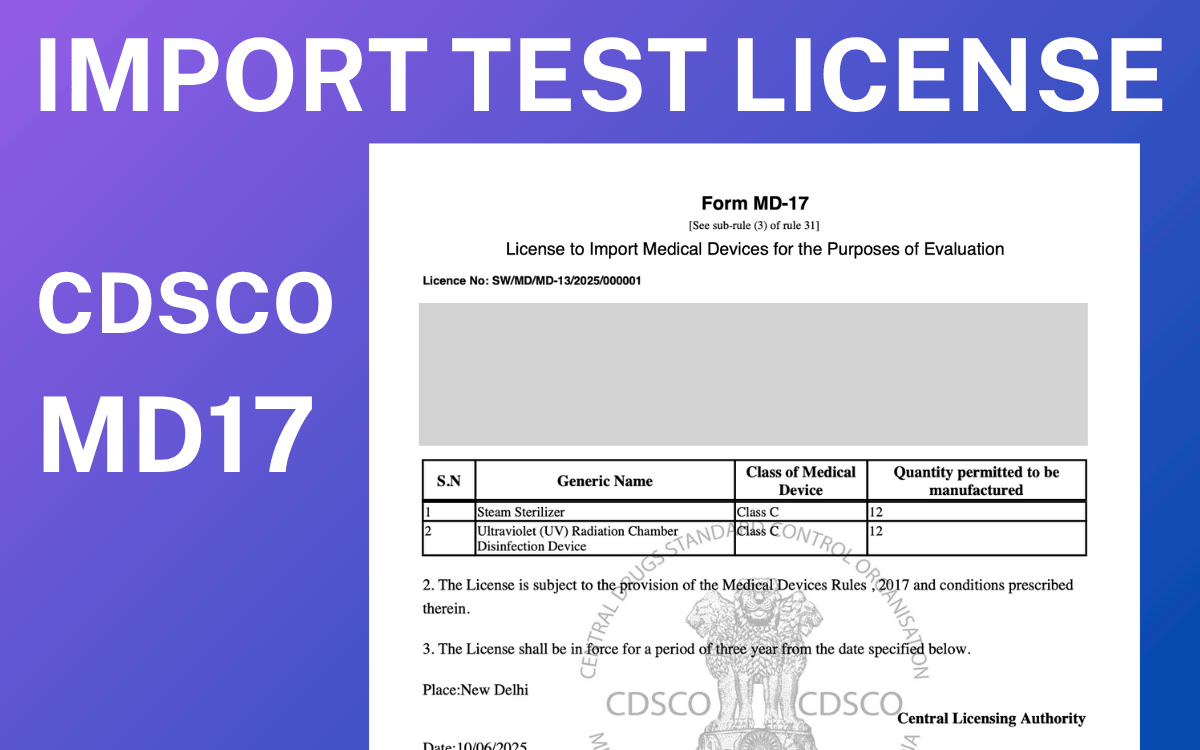
What is the CDSCO MD 17 Test License?
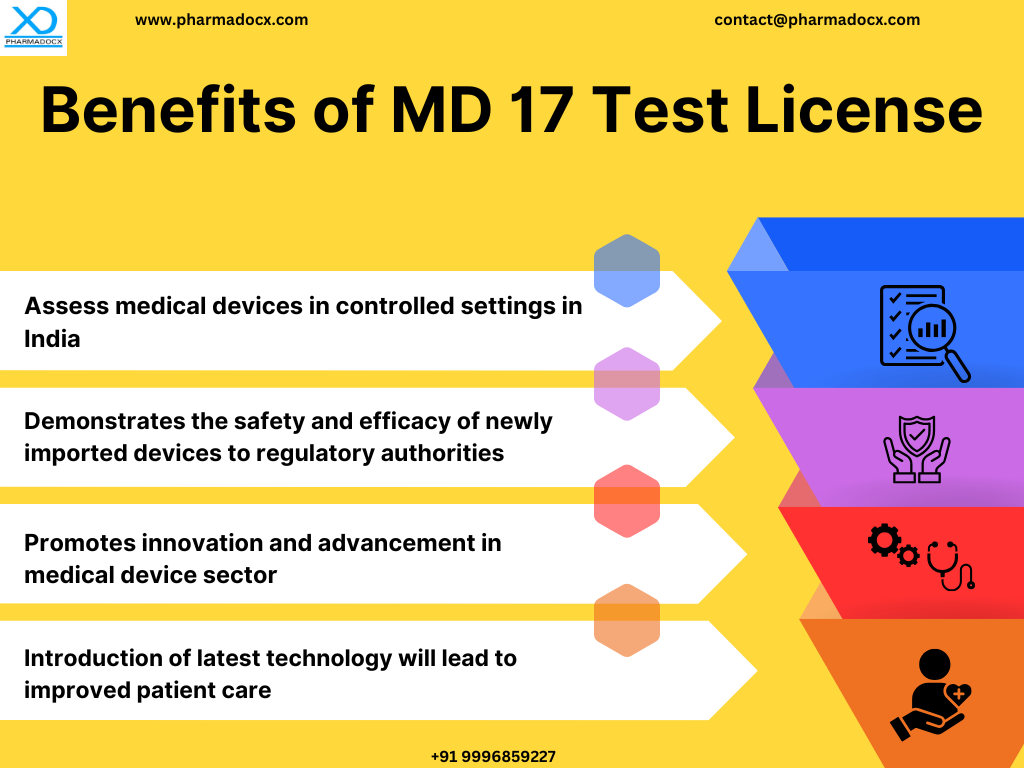
The CDSCO MD 17 test license permits the import of small quantities of medical devices for the purpose of testing, evaluation, training, or demonstration. This license can be used for all CDSCO classes of medical devices, A, B, C, and D. The MD 17 license is useful for importers. Importers can conduct necessary evaluations and assess device performance in the Indian market prior to full-scale import. Additionally, they can check for compliance with Indian safety standards and regulations prior to applying for a full import license. Primary objectives of CDSCO MD 17 test license are:
- Supporting research and development in the medical device sector
- Ensuring the safety and efficacy of imported medical devices are thoroughly evaluated prior to launching them in the Indian market.
- Facilitating controlled entry of new technologies in the medical device sector.
Form MD 16
The applicant has to file the application for MD 17 test license under Form MD 16.
MD 17 license
The CDSCO grants the license to import medical devices for testing purpose under CDSCO MD 17 test license.
Scope of CDSCO MD 17 test license
The MD 17 license specifically grants the importation of medical devices for testing and evaluation purposes only and not for commercial sale. Its scope covers the following aspects:
- Allowing importers to import medical devices for testing within India
- Ensuring compliance with Indian medical device quality and safety standards during the device testing phase
- Facilitating the evaluation of device performance in the Indian healthcare context prior to full scale import.
- Having an CDSCO MD 17 test license may ease out the process of obtaining the CDSCO medical device import license (MD 15) for full scale commercial import.
Who can Apply for CDSCO MD 17 License?
Any entity who wishes to import Class A, B, C, or D medical devices for testing purpose can apply for CDSCO MD 17 license.
Licensing Authority
Central Drugs Standard Control Organization (CDSCO) grants the MD 17 test license.
Timeline for Approval of MD 17 License Application
Timeline for securing the CDSCO MD 17 license is approximately 1.5 to 2 months.
Fees Required for MD 17 License Application
The fee for MD 17 license application is $100 per product.
CDSCO MD 17 Test License: 4 Key Requirements
- Detailed device information: The applicant must provide comprehensive information on the medical device. A brief description of the device and its functionality should be provided. Additionally, the intended use of the device should be clearly explained. Also, a justification for the proposed quantity for testing or evaluation should be mentioned.
- Labelling requirements: Proper medical device labelling and instructions for use are essential for the safe and effective use of medical devices. Hence, applicants must adhere to the medical device labelling guidelines. Clear and accurate labelling of the device should be provided. Additionally, comprehensive instructions for use should be mentioned. Moreover, clearly provide appropriate warnings and precautions.
3. Quality certificates and test protocols: Documents proving the device’s reliability and the applicant’s commitment to maintaining high-quality standards throughout the testing process are required. Quality certificates demonstrating compliance with international medical device quality standards will be required. Moreover, test protocols outlining the planned medical device evaluation procedures have to be detailed.
4. Post-licensing responsibilities: Importers have certain responsibilities even after securing the license. Importers are required to maintain detailed records of all imported devices, including manufacturer details, import date, device specifications, and quantity imported. Notably, proper record-keeping ensures compliance with regulatory guidelines as well as facilitates tracking and management of imported devices. Additionally, importers are required to ensure that all imported medical devices comply with the latest Indian safety standards at all times. They are required to adhere to quality management practices and conduct regular quality checks. Furthermore, they are required to promptly report adverse events related to imported medical devices.
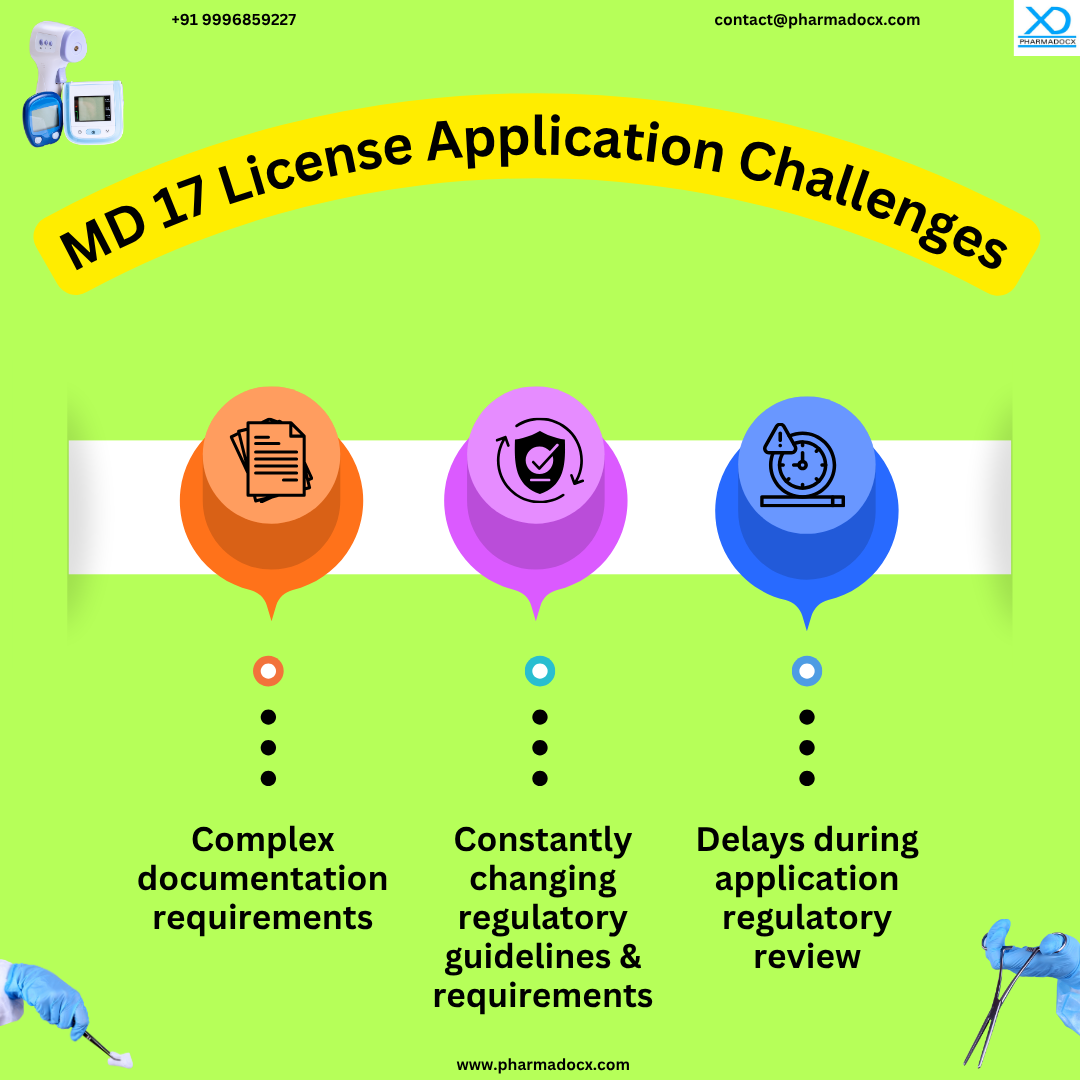
Documents Required for CDSCO MD 17 Test License Application
We have provided an overview of the documents required for MD 17 application.
- Covering letter
- Detailed description of the medical device, including the intended use, design, and the material of construction.
- Justification of proposed quantity to be imported
- Test protocol or approved clinical investigation plan, as applicable
- Quality certificates of the manufacturer (E.g., the quality management system)
- Proper medical device labelling and instructions for use (IFU), including any warnings or precautions, should be clearly provided.
- An undertaking stating the medical devices will be exclusively imported for the purpose specified in Form MD 16 and will not be used for commercial sale is required.
- The place where the demonstration, evaluation, testing, clinical trials, and training will be performed should be specified.
- An undertaking from the testing laboratory stating the laboratory will provide necessary facilities, such as the equipment, instrument, and personnel, to test the imported medical device is required.
- Fees challan
To easily secure MD 17 license, fill out the form below
Contact us
5 Key Points to Remember
The importers who wish to import medical devices for testing purpose should keep the following points in mind:
- A maximum of 10 items are permitted per MD 17 test license application.
- The quantity of medical devices must be specified during the online application process.
- The batch details, amount to be retained, and quantity to be utilized should be specified during the online application process.
- The location where the testing, evaluation, training, or demonstration of the imported medical devices will be conducted must be specified during the online application process.
- If the medical devices will be imported for the purpose of clinical investigation, then the applicant is required to mention the protocol of the clinical trials.
How to Apply for the CDSCO MD 17 Test License?
- Register on SUGAM: Create an account on the SUGAM portal and register your company. Login to the license application portal.
- Fill in the Form MD-16 application: Fill in the application Form MD-16 for CDSCO test license for importing medical devices for testing purposes.
- Upload documents: Upload all the necessary supporting documents per CDSCO MD 17 requirements.
- Pay the application fees: Pay the necessary license application fees
- Submit the application: Submit the application for MD 17 license on the online portal.
- Application review: CDSCO will review the application and verify the documents. They will evaluate the authenticity and completeness of the submitted documents. Additionally, they will check the application for eligibility for the MD-17 license application grant.
- MD 17 license issue: Once all the criteria are satisfied, CDSCO will grant the license. The regulatory body will issue the medical device test license under MD-17 for importing medical devices for testing purpose.
Pharmadocx Consultants Will Help You Easily Secure the CDSCO MD 17 Test License
With the support of our team of experts, you can secure the MD 17 license in a hassle-free manner. Our expertise in document preparation, license application, and regulatory guidelines will make the process seamless. We will ensure you bypass the common license application challenges and have a streamlined regulatory journey. Email at [email protected] or call/Whatsapp on 9996859227 to easily secure the CDSCO MD 17 test license.
Let's Talk!
We'd love to hear from you! Whether you have questions about our pharmaceutical plant setup consultation services or want to discuss a potential project, our team is here to help. Simply fill out the form below, and we'll get back to you as soon as possible. Alternatively, you can reach out to us directly using the phone number or email address listed on this page. We look forward to connecting with you!
Phone / Whatsapp
Address
- Head Office - Opposite Dewan Mill, Old D.C. Road Sonepat - 131001 Haryana, India
- Registered Office - Netaji Subhash Place, Delhi, 110034