CDSCO MD5 License for Class A & B Medical Devices
Easily get CDSCO MD5 License in the shortest time and least hurdles.
For manufacturing Class A & B Medical Devices, CDSCO MD5 License is required. This is granted by State authorities.
Expert consultation with industry leaders
Fast & hassle-free MD5 approval
Manufacturing Registration
Documentation that passes scrutiny the first time
Fast and accurate query replies
600+ Granted Approvals 27+ Years Experience
300+ Happy Clients












The Central Drugs Standard Control Organization (CDSCO) regulates and controls all medical devices entering the Indian market. Hence, medical device manufacturers will have to secure the CDSCO medical device manufacturing license to manufacture medical devices in India. Thus, compliance with CDSCO regulatory guidelines is mandatory. Navigating the CDSCO regulatory guidelines and understanding the license application process can be tricky. Moreover, the CDSCO has categorised medical devices into various classes. Different medical device manufacturing licenses are applicable for the different CDSCO medical device classes. Depending on the CDSCO class to which their device belongs to, manufacturers will have to secure the license type. MD5 and MD9 are the two types of medical device manufacturing licenses issued. We at Pharmadocx Consultants will help you easily secure the CDSCO MD5 manufacturing license.
CDSCO MD5 Manufacturing License for Class A and B Medical Devices
Our team of experts has extensive knowledge of the medical device industry and CDSCO regulatory guidelines. As a regulatory consultant, we provide CDSCO regulatory assistance and support. We provide a one-stop solution for all the CDSCO MD5 manufacturing license needs of your medical device firm.
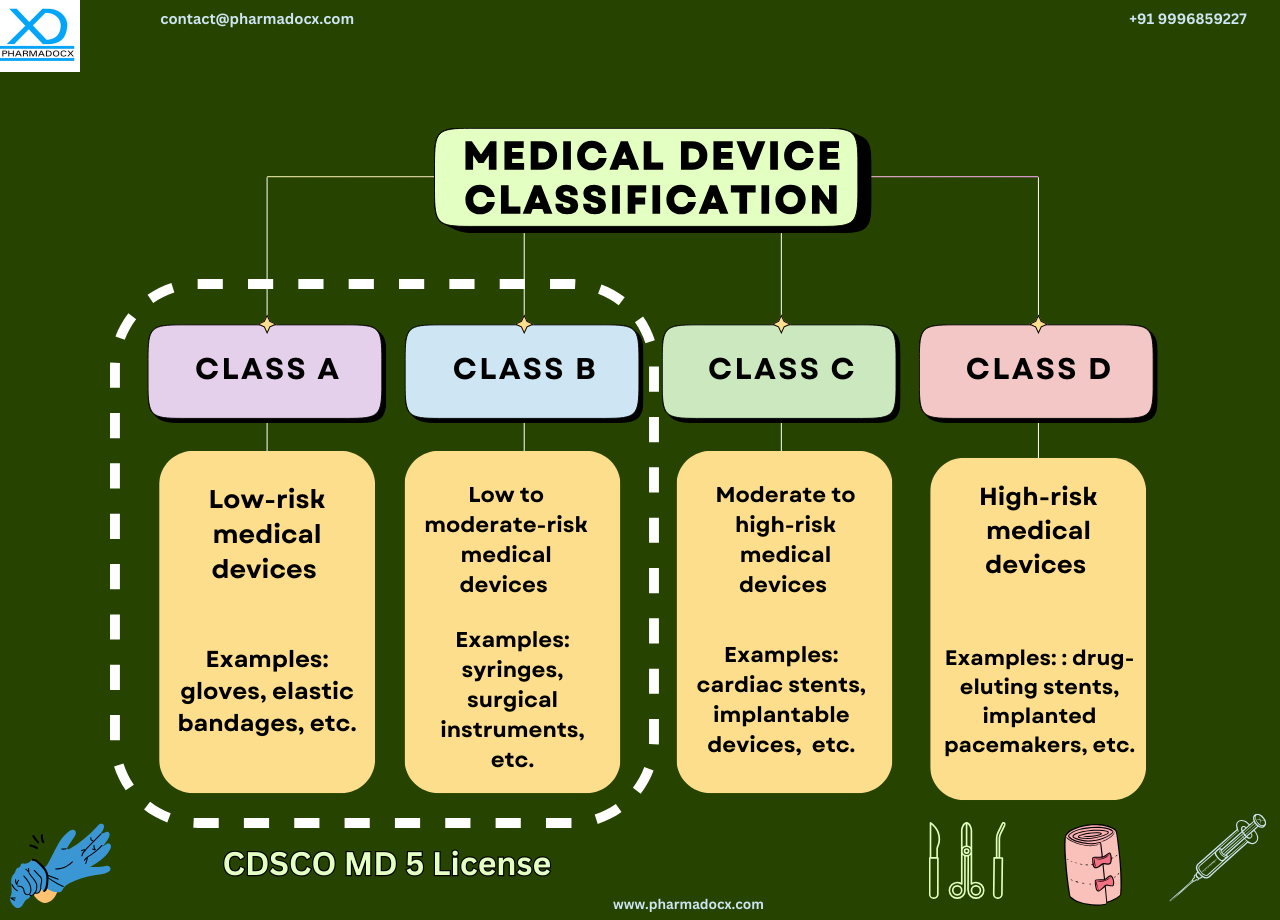
What is the CDSCO Medical Device Classification System?
The first step in CDSCO medical device license application is classifying the device per the CDSCO medical device classification system. The medical device class will determine the license required and the application process. CDSCO classification system has been prepared mainly for the ease of medical device license application. Based on certain parameters, such as risk level and intended use, medical devices are grouped into four classes. The CDSCO medical device classification system is sorted from the lowest to the highest risk. The medical device classification helps determine the regulatory pathway and requirements for market authorization of medical devices.
Furthermore, the manufacturer can fill in the registration and licensing paper work depending on the CDSCO class to which their device belongs to. Additionally, the manufacturer can easily determine which regulatory guidelines will be applicable to their device depending on its CDSCO class. The four CDSCO classes are Class A, B, C, and D.
CDSCO medical device manufacturing license
Securing a CDSCO medical device manufacturing license is mandatory to manufacture medical devices in India. MD 5 and MD 9 are the two primary CDSCO medical device manufacturing licenses. The CDSCO medical device class will determine the license required. Class A and B medical devices require MD 5 license. On the other hand, Class C and D medical devices require MD 9 license.
Medical Device Manufacturing License for Class A and B Medical Devices
MD 3
The application for class A and B medical device manufacturing license is filed under MD 3. The manufacturer will have to submit the Form MD3 to the state licensing authority to secure the license to manufacture class A and B medical devices.
CDSCO MD5 manufacturing license
The CDSCO MD5 manufacturing license grants the permission to manufacture class A and B medical devices in India. The MD 5 license authorises a manufacturer to manufacture class A and B medical devices for sale and distribution in India.
Necessary Supporting Documents for CDSCO MD5 Manufacturing License Application
Certain supporting documents are required for CDSCO MD5 manufacturing license application for class A and B medical devices.
- Sale Deed/Rent Deed proving legal ownership of the manufacturing facility.
- Building layout presenting the dimensions of each room and the location of all equipment in the manufacturing facility. This presents a detailed drawing of the medical device manufacturing facility.
- Organization identity proof, such as UDYAM Aadhar, PAN card, etc.
- Documents proving the manufacturing team comprises competent, qualified, and experienced staff who will manufacture and test the medical devices.
- Plant master file
- Device master file
- Audit reports
- ISO 13485 Certificate validating the manufacturing facility meets international criteria for quality management systems.
- Medical device test license. This will be required in case you need to test your medical devices before launching them in India.
- Certificate of Analysis of 3 Consecutive Batches. This document will certify that the first 3 batches of the manufactured devices meet the required quality benchmarks.
- Document supporting compliance with the environmental regulatory requirements.
The above list provides an outline of the supporting documents required for applying for MD 5 license for class A and B medical devices. For the exact list of documents, feel free to reach out to us.
CDSCO MD5 Manufacturing License Application Support: Our Services
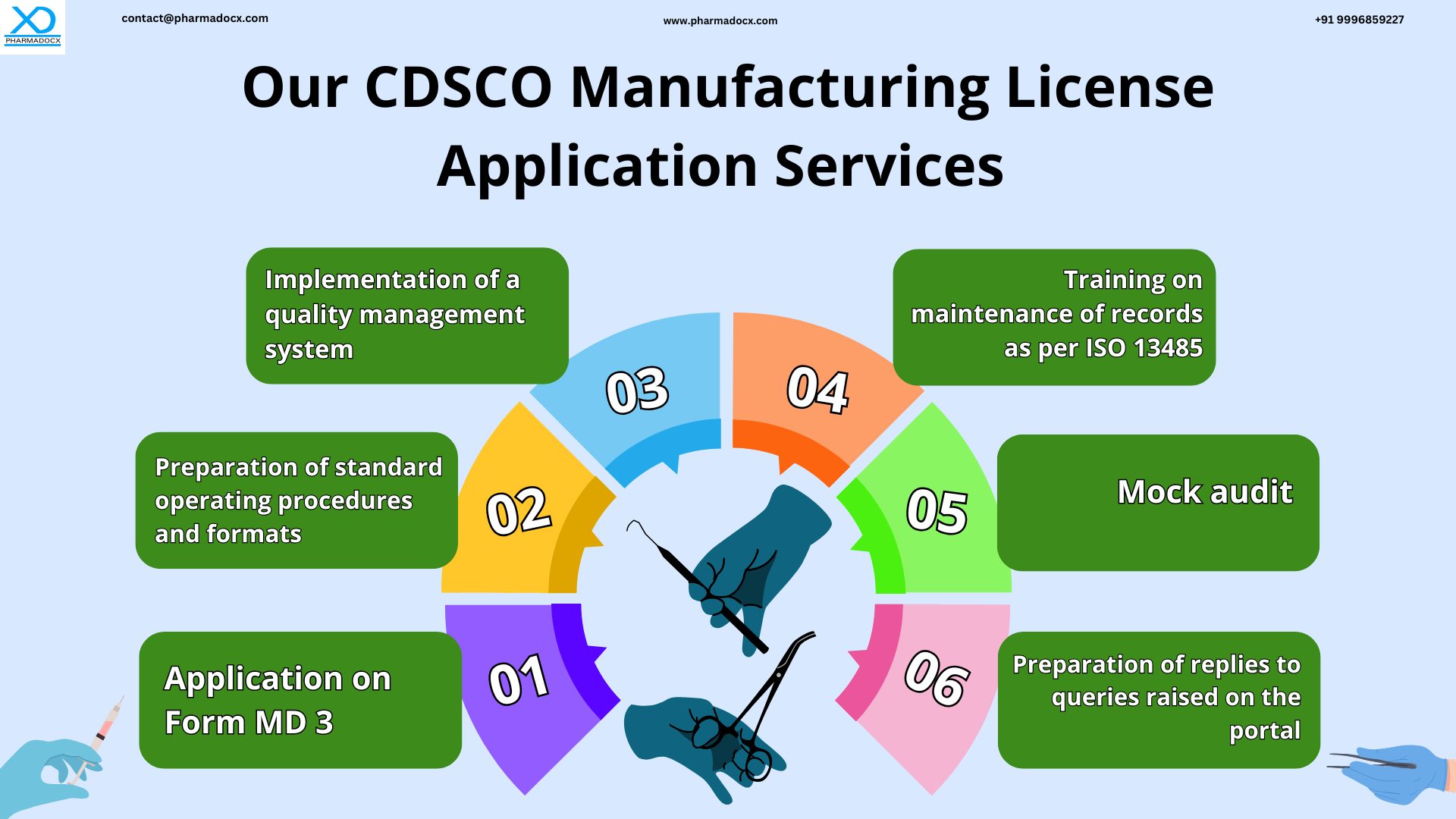
Technical document writing service for CDSCO MD5 manufacturing license application
Technical documents per CDSCO requirements have to be prepared for CDSCO MD5 manufacturing license application for class A and B medical devices. Technical document preparation is a very important aspect of the CDSCO medical device manufacturing license application process. Moreover, not only the documents have to be prepared per guidelines, they have to be arranged accordingly. We at Pharmadocx Consultants will help you prepare and collate all the documents per regulatory guidelines.
Device Master File
The device master file usually contains information about the design and composition of the medical device, materials used in manufacturing, and manufacturing process itself. Additionally, it may also include data on the device’s performance, clinical studies, and adverse event reports. This document has to be submitted to the regulatory authorities to provide information about the safety and effectiveness of the medical device.
The purpose of the device master file is to allow the manufacturer to provide vital information to regulatory authorities without disclosing confidential information to third parties. The file can help streamline the regulatory approval process for the manufacturer. Additionally, it will protect proprietary information. Furthermore, it will improve market access and facilitate international sale.
The Pharmadocx Consultants team has rich experience in the preparing device master files. We will leverage our expertise to prepare the documents per Medical Device Rules, 2017 and ISO 13485:2016.
Plant Master File
A plant master file is a comprehensive document that provides detailed information about a medical device manufacturing facility. It is typically created by the manufacturer or distributor. The file serves as a valuable resource for regulatory authorities evaluating the safety, quality, and efficacy of the medical device.
Need help applying for MD5 license?
Fill out the form below
The plant master file contains detailed information about the manufacturing process, including the facilities, equipment, and personnel involved. Additionally, it also includes information about the quality control systems in place, such as testing methods, specifications, and procedures for handling deviations and non-conformities.
By providing all the necessary information in a standardized format, the plant master file can help streamline the regulatory approval process. Moreover, it can reduce the time and resources required for regulatory review. Avail our comprehensive plant master file preparation service.
Quality management system implementation for CDSCO MD5 manufacturing license application
QMS is one of the vital requirements for manufacturing medical devices. Mainly two standards are followed for the quality management system.

Quality Management System as per ISO 13485:2016
ISO 13485 is an international standard that sets out requirements for a quality management system (QMS) in the medical device industry. It is designed to ensure that medical devices are consistently manufactured and controlled to meet the needs of customers and regulatory requirements.
Having a QMS per ISO 13485 eases the process of obtaining MD 5 license for Class A and B medical devices. The standard covers all aspects of the product lifecycle, from design and development to production, installation, and servicing. Additionally, it also includes requirements for risk management, documentation, traceability, and validation.
ISO 13485 is intended to provide a framework for medical device manufacturers to develop and implement a QMS that is effective in ensuring the safety and performance of their products. Moreover, it is also intended to help companies meet regulatory requirements in different markets around the world.
Compliance with ISO 13485 is voluntary. However, compliance with these guidelines can help speed up the CDSCO MD5 manufacturing license application process.
Quality Management System as per Medical Device Rules, 2017
Quality management system has also been defined in Schedule V of the Medical Device Rules, 2017. The requirements of the QMS as per Schedule V and ISO 13485:2016 are almost similar. However, the difference is in clause numbers and specific requirements for sterile and implantable medical devices. The audit which is conducted prior to the grant of MD 5 license to manufacture Class A and B medical devices evaluates the effectiveness of the QMS.
Our team can help you prepare an effective Quality Management System for compliance with both ISO 13485:2016 requirements and Medical Device Rules, 2017.
Mock audit service for grant of CDSCO MD5 manufacturing license
Prior to the grant of CDSCO medical device manufacturing license, an audit is conducted by the notified bodies or the CDSCO medical device regulatory officers. Our team will help you prepare for the audit conducted by both the notified bodies and state licensing authorities.
We will help you in preparing the documents and conduct a mock audit prior to the actual audit conducted by the officials. We not only help you identify non-conformities but also provide actionable steps to correct them. Additionally, we will guide your technical team to handle queries asked during audits.
Application for CDSCO MD5 manufacturing license for class A and B medical devices
The application for MD 5 license for class A and B medical devices has to be made on the official online portal. Navigating the portal, filling the application form (MD 3), and uploading the documents can be a tricky task. With the support of our experts, CDSCO MD5 manufacturing license application process will be seamless.
We will help you apply for the CDSCO MD-5 license in a hassle-free manner. From preparing the documents and uploading them to filling up the application form will be our responsibility. With our support, your CDSCO medical device manufacturing license application process will be a cake walk.
Why choose Pharmadocx Consultants?
Medical Device Licences
Years Experience
Plants Set-up
Our Clients

Why Should you Choose Pharmadocx Consultants as your regulatory partner?
- Documentation Support: With in-depth knowledge of the required documentation, we assist our clients in preparing all necessary documents. Our documents are accurate and in the correct format per regulatory requirements.
- Time-efficient Approach: We understand the value of time in the medical device industry. Our streamlined processes, backed by years of experience, ensure that your license application is processed in the shortest time frame possible.
- Client-Centric Services: Our clients are our top priority. We tailor our services to meet client needs.
- End-to-End Assistance: From initiating the applicant registration to license approval, we will be with you at every step of the journey.
- Application Progress Updates: We provide regular updates on the status of your application, ensuring you are always in the loop.
- Post-License Support: Our support does not end with the license approval. We offer post-license approval support. We assist with license renewals and any regulatory changes that might affect your business.
Our team is well-versed with the nuances of CDSCO guidelines for medical devices. We ensure that your application aligns perfectly with the regulatory requirements, increasing the chances of swift approval. Call/Whatsapp us at 9996859227 or write to us at [email protected] for a hassle-free CDSCO MD5 manufacturing license application journey.
Let's Talk!
We'd love to hear from you! Whether you have questions about our pharmaceutical plant setup consultation services or want to discuss a potential project, our team is here to help. Simply fill out the form below, and we'll get back to you as soon as possible. Alternatively, you can reach out to us directly using the phone number or email address listed on this page. We look forward to connecting with you!
Phone / Whatsapp
Address
- Head Office - Opposite Dewan Mill, Old D.C. Road Sonepat - 131001 Haryana, India
- Registered Office - Netaji Subhash Place, Delhi, 110034

