CE Marking for Medical Devices
To enter the EU market, CE mark is a mandatory certification for medical devices and IVDs under the EU MDR and IVDR
Expert consultation with industry leaders
Fast & hassle-free CE certification
Documentation that passes scrutiny the first time
Fast and accurate query replies
100+ Granted Approvals 27+ Years Experience
The CE mark is a mandatory certification for medical devices and IVDs under the EU’s Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR). To obtain CE mark under EU MDR and IVDR, manufacturers must meet rigorous requirements related to safety, performance, quality systems, and post-market obligations. These requirements vary by device class and risk level. CE mark under EU MDR and IVDR signifies that a medical or IVD complies with the essential safety, performance, and regulatory requirements. Navigating the complexities of securing the CE mark is a challenging task. It can be a huge ordeal for new entrants in the medical device and IVD sector. With the help of the Pharmadocx Consultants team, you can easily secure the CE marking certification. We specialize in guiding manufacturers through every step of the CE marking process. Our team will guide you through risk classification, technical documentation, performance evaluation, and obtaining the certification.
What is CE Mark?
“CE” denotes Conformité Européenne, which directly translates to “European Conformity.” CE certification is a formal declaration by a manufacturer that their product complies with all applicable EU regulatory requirements. CE marking is a mandatory conformity mark for medical devices (under EU MDR) and IVDs (under EU IVDR). It indicates that the product:
- Meets General Safety and Performance Requirements (GSPRs)
- Has undergone appropriate conformity assessment procedures
- Is backed by a valid EU Declaration of Conformity (DoC)
The aim of CE marking for medical devices and IVDs is to confirm that the product is safe for the intended patient or user population, performs as intended, and complies with all EU regulatory requirements. After receiving the CE marking, the device can be legally marketed and distributed within the European Economic Area (EEA). Having the CE mark confirms the product has successfully undergone the necessary regulatory assessments. Hence, it can be marketed across Europe without the need for additional national approvals.
3 Key criteria for securing the CE mark under EU MDR and IVDR
To secure the CE mark under EU MDR and IVDR, manufacturers must demonstrate compliance in these 3 areas.
- Scientific validity:The medical device should achieve its intended purpose based on established scientific principles.
- Analytical performance:The accuracy, sensitivity, precision, and specificity of the device are evaluated. Analytical performance tests are vital for laboratory instruments, diagnostics, and monitoring devices.
- Clinical performance:The device should be able to deliver the intended clinical benefit when used as directed. For high-risk medical devices, clinical investigations have to be performed to obtain real-world evidences.
What does the CE mark signify?
Importers and manufacturers are required to affix their medical device and IVDs with the CE mark to market their product in EU. Without the CE mark, the medical device cannot be legally placed in the European market. The CE mark signifies the medical device or IVD is:
- Fully compliant with EU MDR or IVDR
- Safe and effective for the intended use
- Ready to be launched within the EEA market
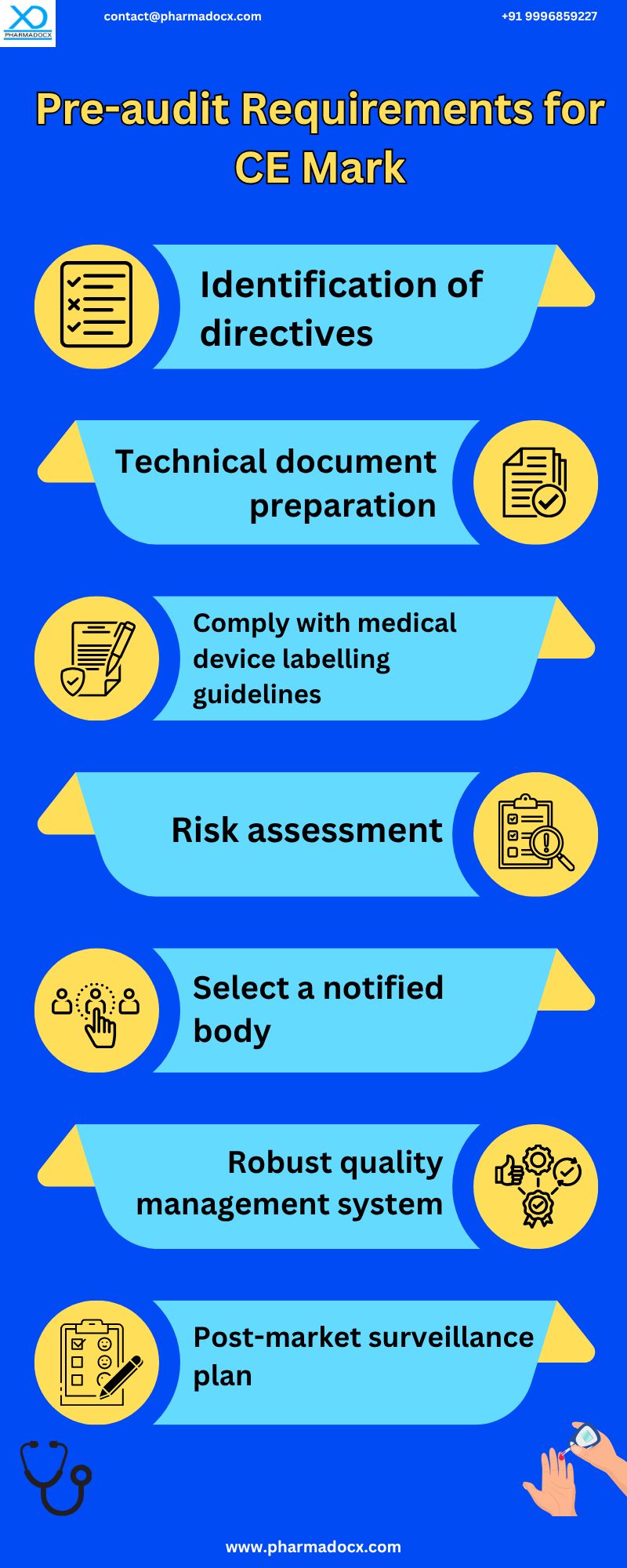
Step-by-Step Guide to Securing the CE Mark Under EU MDR and IVDR
For CE mark certification, the type of product and its specific requirements will determine the level of complexity of the regulatory process. In case of CE mark certification, manufacturer is responsible for ensuring compliance with all relevant EU-wide requirements for the medical device. Additionally, the manufacturer has to determine whether they can assess their product by themselves or whether a notified body will be required. Furthermore, a notified body will demand fees for their service. Moreover, the manufacturer will have to prepare a technical dossier demonstrating compliance.
- Determine applicable regulation: MDR applies to medical devices (e.g., implants, surgical instruments). On the other hand, IVDR applies to in vitro diagnostic devices (e.g., blood glucose meters, COVID-19 tests).
- Classify the device: Medical devices and IVDs are classified based on the risk, intended use, and invasiveness. The MDR classes are I, Ir, IIa, IIb, and III. Whereas, the IVDR classes are A, B, C, and D. The classification determines the conformity assessment route and Notified Body (NB) requirement.
- Implement a quality management system (QMS): A robust QMS will be required for all classes, except lowest-risk devices, to secure CE mark under EU MDR and IVDR. The QMS must comply with ISO 13485:2016 Procedures for design control, risk management, PMS, vigilance, and traceability should be included in the QMS.
- Conduct a conformity assessment: Self-declaration will be required for low-risk devices (e.g., MDR Class I non-sterile; IVDR Class A non-sterile). Notified Body will be required for higher-risk devices. They will focus on the review of technical documentation, QMS audit, and clinical/performance evaluation assessment. Assessment route depends on device class and chosen annex (e.g., Annex IX, X, XI under MDR).
- Compile technical documentation: The device must demonstrate compliance with General Safety and Performance Requirements (GSPRs). The documentation includes device description and specifications, risk management file (per ISO 14971), clinical evaluation (MDR) or performance evaluation (IVDR), labeling and IFU, PMS and vigilance plans, and UDI assignment and traceability.
- Draft the EU Declaration of Conformity (DoC): A legally binding document stating the device complies with MDR/IVDR. This document has to be signed by the manufacturer or authorized representative.
- Affix the CE Mark: Once conformity is confirmed, affix the CE mark on the device, packaging, and IFU. Additionally, include the Notified Body number if applicable (e.g., CE 2797). Notably, the CE mark must be visible, legible, and indelible.
Quality Management System (QMS)
Medical device Quality Management System (QMS) governs the design, development, production, and post-market activities of medical devices. QMS ensures regulatory compliance, promotes continuous improvement, and facilitates product safety and effectiveness throughout the device lifecycle.
EU Authorized Representative
Manufacturers located outside the European Union must appoint an EU Authorized Representative to legally distribute their medical devices within the EU market. This representative serves as a liaison between the manufacturer and EU authorities. Key responsibilities include maintaining technical documentation, managing regulatory correspondence, and facilitating market access under the EU MDR framework.
Medical Device Testing
Medical device testing is a rigorous validation process that confirms a device’s safety, performance, and conformity with applicable international standards. Testing focuses on functional, mechanical, electrical, and biocompatibility assessments. It provides critical evidence of reliability and regulatory readiness. Moreover, it ensures the device is fit for its intended use and eligible for market authorization.
Clinical Evaluation
Clinical evaluation is the systematic process by which manufacturers collect, assess, and present clinical evidence to confirm that a medical device meets the GSPRs. This evaluation ensures the device performs as intended and aligns with regulatory expectations. By demonstrating clinical benefit and safety, it supports regulatory approval and enhances market credibility.
EUDAMED Registration
The European Database on Medical Devices (EUDAMED) is a centralized digital platform established by the European Commission. All medical devices along with manufacturers, importers, or authorised representative have to be registered on the EUDAMED, which is necessary for CE mark under EU MDR and IVDR. EUDAMED is designed to enhance regulatory transparency, device traceability, and market surveillance. It serves as a critical infrastructure for ensuring compliance, safeguarding public health, and reinforcing patient safety across the EU.
Technical File
Technical file is a necessary requirement for securing the CE mark under EU MDR and IVDR. The technical file is a structured compilation of documentation that medical device manufacturers are required to develop and submit to a Notified Body (NB) or Competent Authority (CA). It supports the device’s conformity with applicable regulatory standards, thereby demonstrating its safety, performance, and effectiveness. The file encompasses design specifications, verification and validation data, risk management outputs, and evidence of regulatory compliance.
UDI Labelling
The Unique Device Identifier (UDI) system assigns a globally standardized alphanumeric code to medical devices, thereby enabling precise identification and traceability throughout the supply chain. This harmonized framework supports regulatory compliance, facilitates post-market surveillance, and strengthens patient safety. It ensures accurate device tracking across international jurisdictions and throughout the product lifecycle.
Post-Market Surveillance (PMS)
Post-market surveillance is a proactive and continuous mechanism for monitoring medical devices once they are marketed. It enables manufacturers to detect emerging risks, validate ongoing performance, and implement corrective actions when necessary. PMS is essential for sustaining regulatory compliance and improving product quality.
Time Required for Securing the CE Mark Under EU MDR and IVDR
The CE mark certification process takes approximately four to six weeks for approval. However, the approval process can take longer, if the product needs modification or the technical documentation is not fully complete.
Validity of CE Mark
The CE marking certification is usually valid for a period of three years. After this period, the certification has to be renewed. Notably, it is recommended to start the registration renewal process at least 6 months prior to the expiry of the certification.
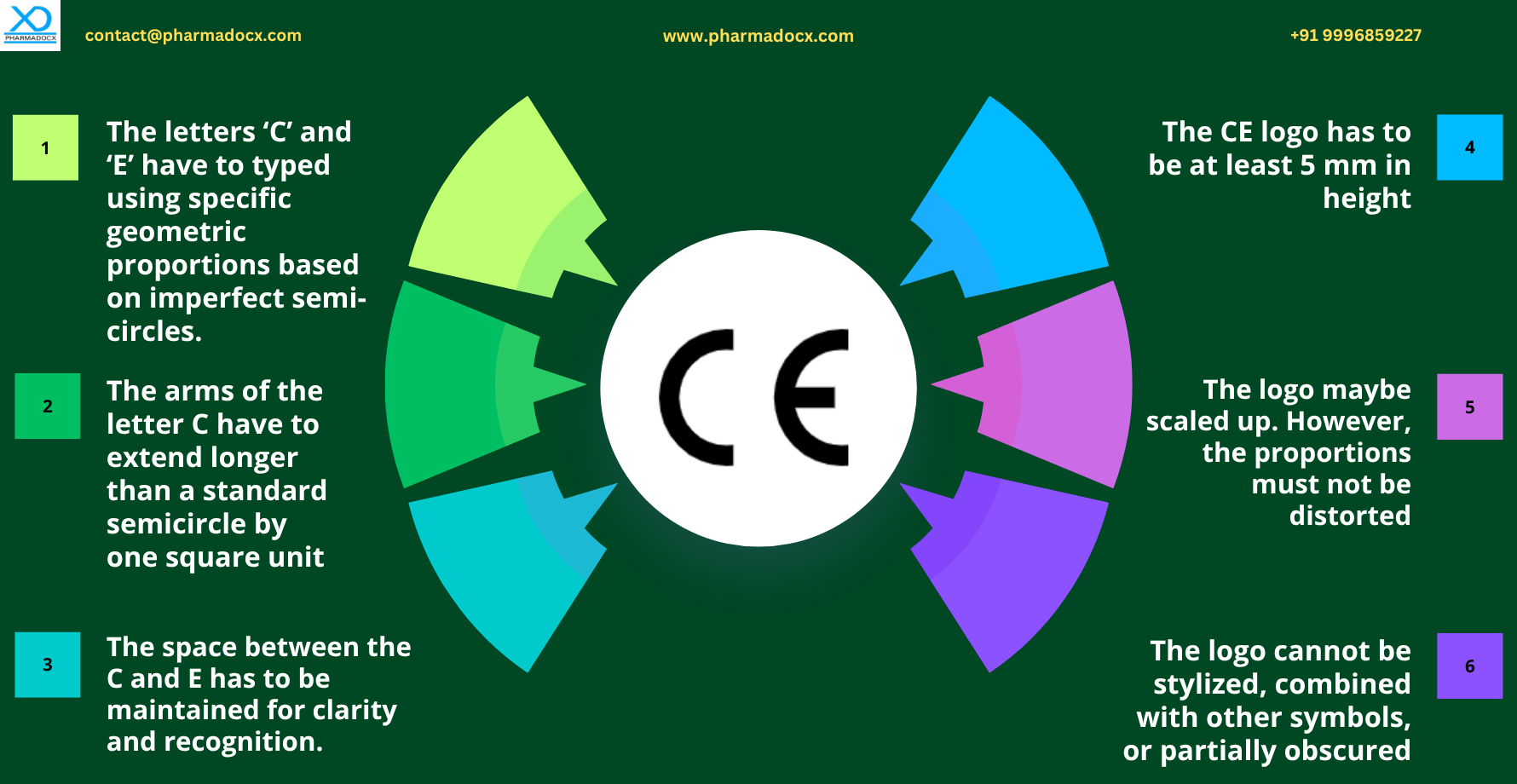
Design Standards for the CE Logo
The CE mark under EU MDR and IVDR has strict design standards. Moreover, modifying the CE logo is not permitted. We have listed the important design and size guidelines:
Where Should the CE Mark Be Placed?
The CE logo must be visible to the user and authorities. Additionally, it should be legible and easily identifiable. Also, the logo should be indelibly affixed so that it cannot be removed or tampered with. The acceptable locations are as follows:
- Directly on the product (preferred)
- On the data plate or nameplate
- On the outer packaging
- In accompanying documents like user manuals or instructions for use (IFU)
5 Common Mistakes to Avoid While Using CE Marking
Notably, medical devices and IVDs with an incorrect or missing CE logo may be banned from entering the EU market. Penalties or recalls may be imposed and distribution maybe blocked. Hence, we have listed some common mistakes which you should steer clear off.
- Using a fake or altered CE logo should be completely avoided
- Affixing the CE mark prior to completing conformity assessment
- Skipping technical supporting document preparation or Declaration of Conformity (DoC)
- Not consulting with a notified body while applying for CE marking for high-risk devices
- Failing to register in the EUDAMED, which is the European database for device registration
Need help securing the CE mark certification?
Fill out the form below and we will get back to you
End-to-end Support for Medical Device and IVD CE certification
Our CE marking consultants help manufacturers place their devices in a hassle-free manner in the European Market. We provide customised services to meet your regulatory needs.
How can Pharmadocx Consultants Help You Easily Secure the CE Mark For Medical Devices and IVDs?
Our team will help you in the following ways:
- Identification of applicable EU directives for your device
- Verification of applicable standards and testing requirements
- Compile and prepare necessary supporting and technical documents
- Reviewing marketing materials, labels, and user manuals for compliance and consistency
- Ensuring adherence to essential requirements
- Implementing, adjusting, and maintaining a robust quality management system (usually ISO 13485) to meet European standards
- Conducting risk assessment and management per applicable guidelines
- Acting as a communication bridge between the client and regulatory officials
- Providing application submission guidance and support throughout the process
To secure the CE mark under EU MDR and IVDR, email at [email protected] or call/Whatsapp on 9996859227.
FAQs on CE mark under EU MDR and IVDR
Why is CE mark mandatory?
CE marking is mandatory for all devices intended for European Economic Area (EEA). It indicates the devices are safe for patients and users, perform as intended, and meet all regulatory obligations. This certification builds trust among the healthcare professionals and patients.
How long does CE certification take?
The duration of CE certification depends on several factors, including the risk classification of the device, the completeness of the technical documentation, and the responsiveness of the Notified Body. On an average, Class I devices without Notified Body involvement may take 2 to 4 months. Whereas, medium to high-risk devices (Class IIa, IIb, and III) involving a Notified Body can take 6 to 18 months or longer. Notably, timely preparation of technical files, clinical evaluation, and risk management documents can significantly reduce the timeline. Partnering with Pharmadocx Consultants’ team of experts can help streamline the process and avoid unnecessary delays.
Do all medical devices intended for EU need a CE mark?
Yes, all medical devices intended for sale in EU must have a CE mark. The CE marking shows that the device meets the safety, health, and performance requirements outlined in EU MDR/IVDR. Devices without a CE mark cannot be legally marketed or sold in the EU.
Who needs CE certification?
Any manufacturer who wants to sell medical devices or IVDs in the EU must obtain CE certification. This applies to both EU-based and non-EU manufacturers.
Why should you hire Pharmadocx Consultants for CE certification?
We at Pharmadocx Consultants offer end-to-end consultancy support for CE certification under EU MDR and IVDR. Our experienced consultants will guide you through every stage of the CE marking process, from device classification, risk analysis, and technical documentation to coordination with Notified Bodies and audit preparation. Additionally, we assist with EUDAMED registration, UDI labelling, and post-market surveillance, and ensure your Quality Management System (QMS) aligns with EU standards. With our support you will have a smooth CE certification process, irrespective of whether you are a first-time applicant or renewing your certification.
Let's Talk!
We'd love to hear from you! Whether you have questions about our pharmaceutical plant setup consultation services or want to discuss a potential project, our team is here to help. Simply fill out the form below, and we'll get back to you as soon as possible. Alternatively, you can reach out to us directly using the phone number or email address listed on this page. We look forward to connecting with you!
Phone / Whatsapp
Address
- Head Office - Opposite Dewan Mill, Old D.C. Road Sonepat - 131001 Haryana, India
- Registered Office - Netaji Subhash Place, Delhi, 110034

