Certificate of Pharmaceutical Product:
COPP Certification Service
India is a major pharmaceutical exporter and one of the largest providers of generic drugs. As high-quality affordable medicines are manufactured in India, India has earned the name “pharmacy of the world”. Additionally, a highly skilled resource pool and low labour costs are an added advantage for India. To export the drug, certificate of pharmaceutical product will be required to register the drug in the importing country.
COPP certification service in India
The certificate of pharmaceutical product (COPP) has to be procured in India to export pharmaceutical products to other countries. A COPP certification service makes this process easy.
We at Pharmadocx Consultants will simplify the complex process of securing certificate of pharmaceutical product.
With in-depth knowledge of the WHO regulations and industry quality guidelines, we will help you ensure your pharmaceuticals comply with the applicable regulations.
Our expertise in this domain will make your COPP certification journey a cake walk.
What is certificate of pharmaceutical product?
Certificate of pharmaceutical product (COPP) is required for global marketing approval of a drug. It is usually issued by the National Health Authorities of the exporting country to be used by the importing country. This certification is issued by the exporting country to demonstrate the drug complies with industry quality guidelines and WHO regulations. Moreover, COPP certification indicates the drug is of high-quality and is safe and effective. Notably, this certification is usually issued for a single product not for the pharma company or manufacturing process.
This certificate is issued in a format recommended by the WHO. Only after evaluating the efficacy, quality, and safety of the pharmaceutical product, the certificate is issued. It establishes the status of the drug in the exporting country. To market a drug in another country, the COPP is required to register the drug in the importing country. Thus, COPP is required for registration, licensing, authorization, and license renewal purposes in the importing country.
Common certification format or types:
- WHO 1975 version
- WHO 1988 version
- WHO 1992 version
- USFDA version
The products for which certificate of pharmaceutical product are usually issued are active pharmaceutical ingredients (API), over the counter (OTC) drugs, homeopathic products, etc. This certificate indicates the manufacturer complies with the GMP guidelines. Additionally, it also implies the manufacturing facility are subject to regular inspection by the regulatory body of the exporting country.
The need for COPP certification service in India
India being a major pharmaceutical exporter has a high demand for COPP certification service. To export and market drugs in foreign countries, COPP certification will be required to register them in the importing country.
The Indian pharma industry caters to countries across the world. Moreover, India has more than 3,000 pharmaceutical companies and over 10,500 pharmaceutical manufacturing facilities. Approximately 60,000 different generic brands across 60 therapeutic categories are manufactured in India. India fulfils over 50% of Africa’s and 40% of the US’s demand for generic drugs.
Thus, globally, India is the largest supplier of generic drugs, holding a 20% share in global supply by volume. Moreover, it also supplies approximately 25% of all medicine requirements in the UK.
Globally, India is one of the biggest suppliers of low-cost vaccines, accounting for 60% of global vaccine production. India fulfils approximately 70% of the WHO demand for DPT and BCG vaccines and 90% of the measles vaccine requirement.
Thus, to register, secure licenses, market drugs, and even renew licenses in importing countries, COPP certification will be required. The certificate of pharmaceutical product has to be procured in India to export pharmaceutical products to other countries. Hence, there is a high demand for COPP certification service in India. Our COPP certification service makes the certification process hassle-free.
Necessary documents required for obtaining certificate of pharmaceutical products
These are some of the documents that have to be submitted along with the application for COPP:
- Covering letter
- Manufacturing license
- Plant master file
- Validation plans and formula records
- GMP Certificate
- Product details
- Labelling and packaging information
- Product registration certificate
- Free sale certificate
- Clinical trial data (if applicable)
- Pharmacovigilance data and record
- Stability Data
- Power of attorney (if using a representative)
This list is only an overview of the documents required. For a detailed list of documents, feel free to get in touch with us. Additionally, we provide document preparation support. Avail our document preparation service to easily prepare the necessary documents within the stipulated deadline.
COPP application process
- An application for the COPP certificate addressed to the ADC/DDC has to be presented to the zonal or sub-zonal officer. A cover letter along with the pharmaceutical product details have to be included with the application.
- The application should clearly mention whether the applicant is applying for a new certification or reissue.
- The manufacturing license, plant master file, GMP certificate, and other certifications and documents have to be collated.
- Pharmaceutical product-related information, such as validation plans, formula records, quality control process, etc., have to be provided.
- The regulatory officials will review the application and inspect the facility. The regulatory authorities will check for WHO GMP compliance. They will prepare a report stating their review findings.
- If the necessary criteria are satisfied, then the officials will provide clearance for COPP certification. Then, the certificate of pharmaceutical product will be issued on behalf of Drug Controller General India (DGCI). However, if the officials find any discrepancies or the necessary criteria are not satisfied, the COPP application will be rejected. After correcting the errors and ensuring regulatory compliance, the applicant may apply again within 5 months.
Fill out the form below to secure your COPP in a hassle-free manner
Our COPP certification service can make this complicated application process a breeze. We will support you throughout the process right from cover letter and application preparation to collating the necessary supporting documents. Additionally, we will make your manufacturing facility inspection ready.
Why should you apply for certificate of pharmaceutical product?
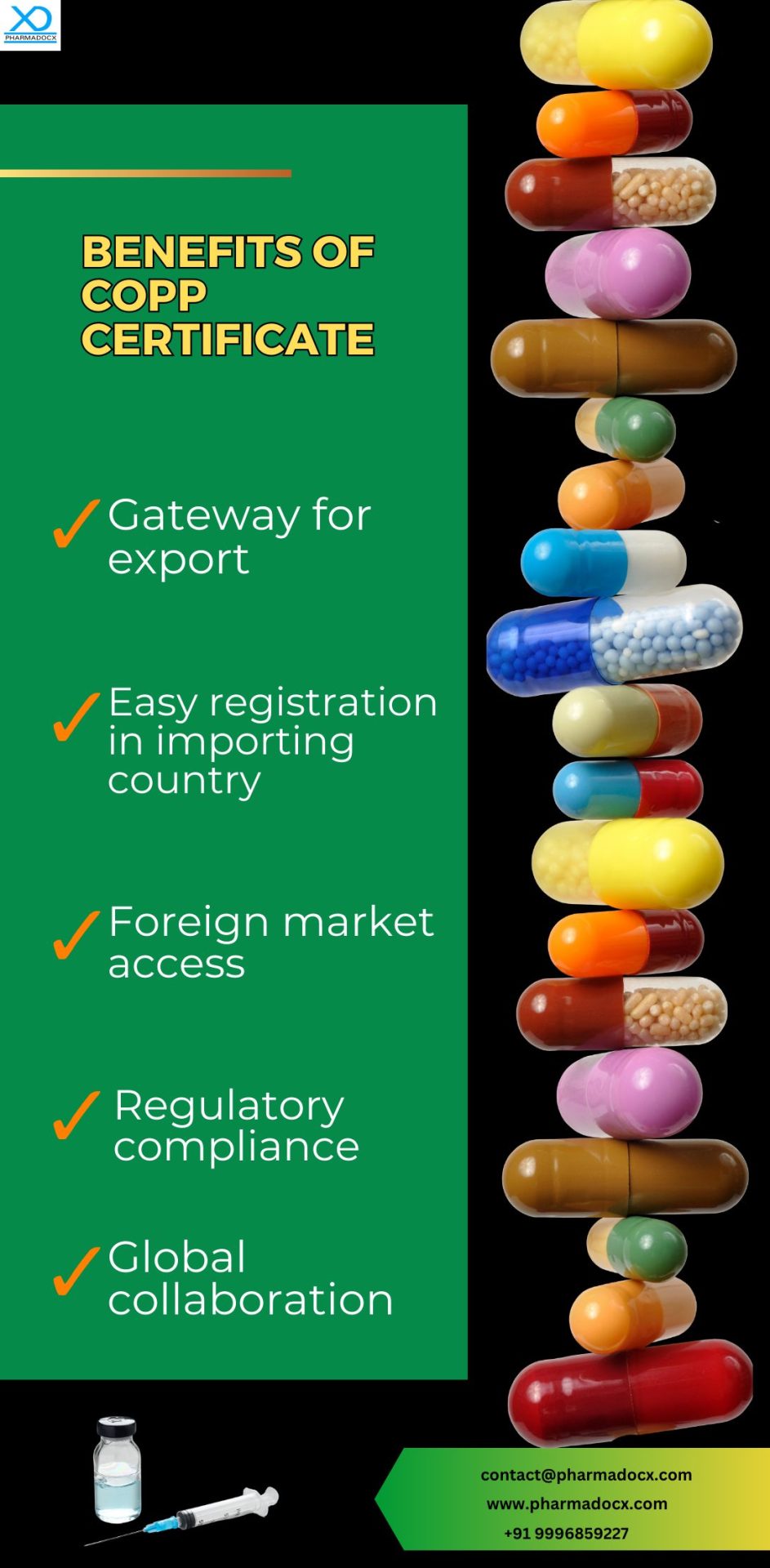
- COPP certification provides access to foreign markets. This certificate will be required to register drugs in foreign countries. It helps streamline the import process.
- To obtain COPP certification, regulatory and GMP guideline compliance are mandatory. Ideally, high-quality, safe, and effective drugs are COPP certified. Thus, patient safety and security are ensured.
- Certificate of pharmaceutical product demonstrates the manufacturer’s commitment to patient safety. Thus, the certificate increases trust on the manufacturer, thereby providing a competitive edge.
- COPP certification will reduce obstacles in the import process.
- COPP certification increases the credibility of the drug in the global market. Thus, COPP leads to global market approval.
Why choose Pharmadocx Consultants?
Drugs Licences
Years Experience
Plants Set-up
Our Clients

How will Pharmadocx Consultants help you secure the COPP certificate?
- We will help you fill up the application and guide you through the entire application process.
- We will help you prepare the necessary documents and collate them per application requirements.
- Our staff training sessions will help your technical personnels manufacture the drugs per GMP guidelines.
- We will provide precise guidance for modifying the manufacturing facility and equipment to make them GMP complaint. Additionally, our team of experts will conduct mock audits to help you prepare for the COPP inspection. Furthermore, we not only identify lapses but also provide corrective actionable next steps.
- Our team will act as a bridge between you and the regulatory officials. By acting as a liaison, we help ensure easy communication between the two parties. Moreover, we help interpret regulatory feedback and assist in preparing responses.
- Our team will track the application process. Additionally, we will provide real time updates.
- We will designate a dedicated expert to assist you throughout the process. We will provide assistance till you successfully secure the COPP.
Pharmadocx Consultants: COPP certification service
Avail our COPP certification service for a seamless experience. Our team of experts will ensure you obtain your certificate of pharmaceutical product in a hassle-free manner.
Let's Talk!
We'd love to hear from you! Whether you have questions about our pharmaceutical plant setup consultation services or want to discuss a potential project, our team is here to help. Simply fill out the form below, and we'll get back to you as soon as possible. Alternatively, you can reach out to us directly using the phone number or email address listed on this page. We look forward to connecting with you!
Phone / Whatsapp
Address
- Head Office - Opposite Dewan Mill, Old D.C. Road Sonepat - 131001 Haryana, India
- Registered Office - Netaji Subhash Place, Delhi, 110034