Effectively Implement Medical Device Risk Management
Medical devices have a vital role in the healthcare industry. As they directly impact diagnosis, treatment, and patient care, medical devices are heavily regulated. Thus, various industry regulations are in place to monitor the quality and efficacy of medical devices. Furthermore, these regulations warrant risk management for medical devices to ensure high quality and effective medical devices are manufactured. Medical device risk management has to be effectively implemented to ensure patient safety and regulatory compliance and improve product reliability. Additionally, industry regulations provide risk management framework and guidelines that manufacturers are expected to abide by.
We at Pharmadocx Consultants provide all-encompassing support for effectively implementing risk management in medical device industry. Additionally, our team of experts has in-depth knowledge of the industry risk management guidelines. Having served over 600 clients and with extensive knowledge of the regulatory guidelines, we are uniquely positioned to help you implement medical device risk management.
What is medical device risk management?
Medical device risk management involves identification, control, and mitigation of potential risks associated with the device. Manufacturers are required to identify possible flaws in the medical device design and manufacturing process that can lead to hazards. Risk management strategies are aimed at creating reliable devices by minimizing the likelihood of failures. Moreover, it plays a crucial role in the product development lifecycle of medical devices. The primary goal is ensuring safety of patients, operators, and the environment. Furthermore, risk management for medical devices focus on ensuring the dependability and proper functioning of the devices. Regulatory bodies mandatorily require manufacturers to incorporate risk management strategies while developing medical devices. ISO 14971:2019 is one of the primary industry regulations presenting structured and detailed medical device risk management framework and guidelines.
Furthermore, manufacturers are required to record all risk management activities in a comprehensive risk management document. The document should detail risk analysis, evaluation, and control of risks. Additionally, it should present the verification of implemented measures and assessment of residual risks.
What are the major elements of medical device risk management?
We have presented some of the key elements that form the basis of risk management for medical devices.
Risk analysis
Proper risk assessment and analysis is the foundation of effective risk management for medical devices. Both unknown and known foreseeable risks have to be considered. All possible risks associated with the medical device, considering all potential hazards, have to be analysed.
Risk evaluation
Estimated risks for each hazardous situation have to be thoroughly evaluated based on the risk management plan and criteria. If the risk is unacceptable, risk control measures have to be implemented. However, if the risk is within the acceptable limit, further risk control activities are not required.
Risk control
After analysing and evaluating the potential risks associated with the medical device, risk control measures have to be implemented. Design modifications, warnings, training, protective mechanisms, and user instructions are all a part of risk control measures. These measures have to be designed to mitigate the identified risks.
Analysis of the risk–benefit ratio
Residual risks have to be weighed against the benefits of using the medical device. The risk–benefit ratio analysis helps determine whether the benefits outweigh the risks of using the medical device. Thus, whether the medical device provides a net positive impact on the patient’s health can be analysed.
Evaluation of overall residual risks
After implementation of risk control measure, residual risks have to re-evaluated for their acceptability with respect to the benefits offered by the medical device. Moreover, the criteria defined in the risk management plan should be used to evaluate the remaining risks. If the overall residual risk is deemed acceptable, users must be informed about the remaining risks. Additionally, manufacturers must provide necessary information to users for informed decision-making.
Review of medical device risk management
Medical device manufacturers should thoroughly review their medical device risk management strategies. They should check whether the risk management plan is being properly implemented. Furthermore, the acceptability of the overall residual risk level needs to be checked. Additionally, appropriate methods to collect and review information during the production and post-production phases should be in place.
Continuous monitoring
The medical device’s performance and safety should be regularly monitored and reviewed. This will help collect new information on the device’s safety and effectiveness. Furthermore, this regular assessment will provide information for any necessary timely modifications required in the risk management strategies.
Documentation of risk management for medical devices
Manufacturers are required to record all risk management activities in a comprehensive risk management document. Notably, clear and accurate documentation helps with regulatory compliance, traceability, and accountability. The document should be used to record risk assessments, evaluations, decisions, and measures implemented.
Clear and effective communication
There should be clear communication among all parties involved in the medical device’s development, manufacturing, distribution, use, and post-market surveillance. Furthermore, risks, precautions, and instructions for use should be clearly communicated to patients and healthcare professionals.
Regulatory compliance
Medical device regulatory guidelines demand thorough medical device risk management prior to marketing them. Thus, medical device manufacturers have to perform effective risk management per the guidelines and frameworks provided by industry regulations. Furthermore, they have to ensure the risk management process complies with applicable regulatory standards and guidelines, such as ISO 14971.
Review and analysis of production and
post-production activities
Production and post-production activities have to be reviewed to promptly identify and resolve potential risks throughout the medical device’s lifecycle. Moreover, a system has to be developed to actively collect and review information during production and post-production phases of the medical device. When developing this system, manufacturers should incorporate suitable methods for effectively collecting, processing, and analyzing information. This information will provide insights into the medical device’s performance, safety, and potential risks throughout its lifecycle.
Why choose Pharmadocx Consultants?
Medical Device Licences
Years Experience
Plants Set-up
Our clients

Why should you incorporate risk management in medical device industry?
- Risk management is vital for maintaining product quality and efficacy. Effective risk management strategies for medical devices will help identify potential risks/vulnerabilities in the device’s design and manufacturing process. Then, the measure can be implemented to address these risks at an early stage. Risk management helps medical manufacturers ensure their devices meet quality standards and perform as intended. By incorporating risk management in medical device industry, manufacturers can improve product quality, reliability, and performance.
- Regulatory bodies, such as the FDA and CDSCO, require medical device manufacturers to abide by specific risk management regulations. Thus, medical device manufacturers have to effectively implement medical device risk management strategies for obtaining regulatory approvals.
- Incorporating risk management in medical device industry can foster an environment of continuous improvement. This can help manufacturers to adapt to new challenges and advances in the field of medical devices.
- Improved patient outcome and protection of patient safety are the primary reasons for implementing risk management in medical device industry. Any failure or malfunction of the medical could have detrimental effects. Thus, medical device risk management strategies have to be effectively implemented to identify potential risks and mitigate them to ensure patient safety.
Want to implement risk management in your medical device company?
Fill out the form below to let us help you
5. Effective risk management strategies help reduce waste of time and resources spent on unnecessary risk mitigation efforts. Well-planned strategies enable medical device companies to allocate resources more efficiently by focusing on high-priority risks. Furthermore, effective risk management strategies can help prevent loss of resources spent on recalls, redesigns, and other costly corrective actions. Moreover, addressing issues at an early stage is more cost-effective than addressing them at the production or post-marketing stage.
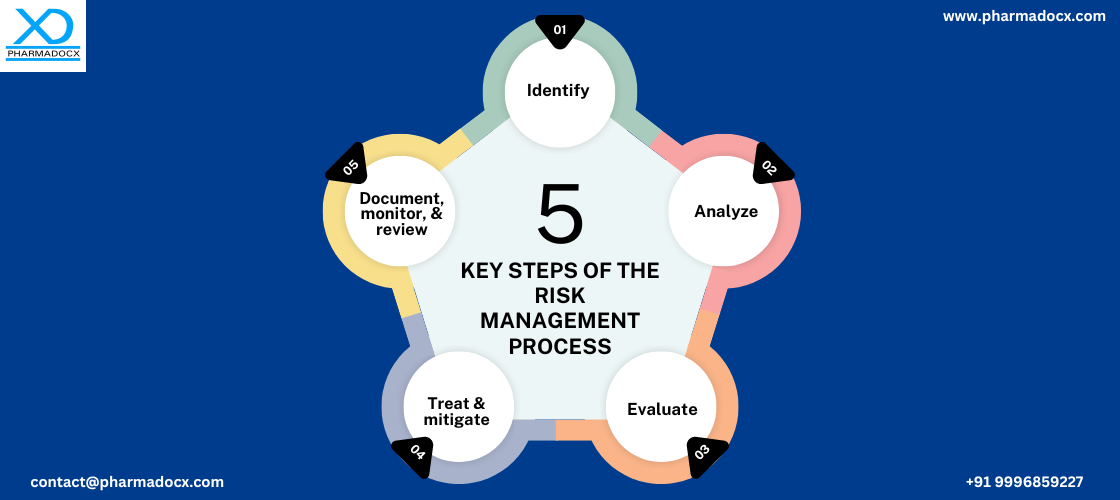
Medical device risk management process
A risk management process as per industry regulations has to be developed. Additionally, the process should be properly documented so that it can be effectively implemented. Moreover, the roles and responsibilities of various parties should be mentioned in this risk management dossier.
Pharmadocx Consultants team will help you effectively implement risk management in your medical device company
We at Pharmadocx Consultants provide comprehensive support for effectively implementing medical device risk management. Additionally, we provide documentation support. Notably, documentation is vital for regulatory compliance and accountability. Our team will help you manufacture medical devices with the highest safety and performance standards by incorporating risk management strategies in your production process.
Let's Talk!
We'd love to hear from you! Whether you have questions about our pharmaceutical plant setup consultation services or want to discuss a potential project, our team is here to help. Simply fill out the form below, and we'll get back to you as soon as possible. Alternatively, you can reach out to us directly using the phone number or email address listed on this page. We look forward to connecting with you!
Phone / Whatsapp
Address
- Head Office - Opposite Dewan Mill, Old D.C. Road Sonepat - 131001 Haryana, India
- Registered Office - Netaji Subhash Place, Delhi, 110034