Implementing Medical Device Quality Management System (QMS)
The medical devices sector is a highly regulated industry with focus on quality required at every stage of production. This sector is stringently regulated and monitored due to its direct impact on patient safety and health. A quality management system (QMS) specifically designed for this sector is required to address the complex product development lifecycle. Moreover, maintaining safety and quality standard compliance is a considerable issue in the medical device manufacturing industry. Medical device quality management system ensures product efficacy and safety. Hence, medical device manufacturers deploy quality management system for medical devices to ensure standardization in order to comply with regulations. Medical device QMS helps improve product quality and safety, standardize processes and documentation, and enhance operational efficiency.
What is a medical device quality management system?
The medical device quality management system is a structured set of procedures, policies, and processes implemented by the manufacturer throughout the medical device’s lifecycle. The medical device QMS covers all aspects of medical device production from design, manufacturing, product labeling, storage, distribution, and supplier management to risk management, complaint handling, and clinical data collection. The aim of QMS is improving and maintaining the quality of medical devices. Additionally, it helps manufacturers constantly and consistently meet customer expectations and regulatory requirements. In short, the quality management system for medical devices is a structured system that documents the procedures and processes throughout the medical device’s lifecycle.
Medical device quality management system encompasses the following aspects
- Medical device design and manufacturing process
- Process and production quality control, including manufacturing equipment surveillance as well as risk monitoring
- Management responsibilities and duties, including readiness, review, and audit
- Change control
- Quality audits
- Resources for staff training and work environment
- Identification of non-compliance
- Complaint handling
- Corrective and preventive action
The medical device quality management system is essential to ensure quality in every stage of medical device production from developing, manufacturing to distributing products. The aim of medical device QMS is to ensure the products being manufactured are safe and will function as intended.
Essential components of medical device QMS
Medical device manufacturers should implement robust QMS to ensure patient safety and protection of public health. Thus, the medical device QMS has to be well rounded and all encompassing. Moreover, it has to contain certain essential components to be effective. We have listed some of the essential components:
- Compliance with industry standards and regulatory requirements has to be at the core of medical device quality management system.
- Effective design and development, rigorous testing, and validation procedures are some of the essential parameters to consider while establishing QMS.
- A rigorous document control system is also required.
- Additionally, risk management for identifying and mitigating potential issues is also necessary.
- Furthermore, employee training and adherence to best quality practices form a part of comprehensive medical device QMS.
- Post-market surveillance is also an integral component.
- Finally, handling consumer complaints, addressing customer feedback, and implementing corrective and preventive action are also essential medical device QMS components.
Quality management system for medical devices: A vital requirement for manufacturing safe and effective devices
The medical device QMS is a structured framework established to consistently manufacture safe and effective medical devices. QMS covers processes, procedures, and documentation control to help manufacturers meet industry and regulatory requirements. Moreover, an effective medical device QMS will focus on product development, manufacturing, risk management, and post-market surveillance. Additionally, an effectively implemented QMS will lead to the production of high-quality, effective, and safe medical devices.
ISO 13485 and FDA 21 CFR Part 820 are some of the regulations for quality management system for medical devices. Furthermore, not just medical device manufacturers, medical device raw material suppliers are also required to adhere to these regulations. This is because the quality of raw materials will affect the quality of the finished product. These international regulations for medical device QMS provide parameters to evaluate the medical device manufacturer’s quality management system. Moreover, these regulations assess the effectiveness of the quality management system in place, regulatory compliance as well as customer satisfaction.
Contact us to effectively implement QMS in your medical device company
Why should manufacturers implement medical device quality management system?
- Medical device QMS helps evaluate key quality and supply chain metrics and provides quality insights into the manufacturing process.
- It minimizes manufacturing defects.
- It helps achieve operational excellence and lower production and operation costs.
- Medical device quality management system helps continuously assess the raw material quality.
- It is a closed-loop system that tracks quality at every stage of the manufacturing process.
- Medical device quality management system can be used to identify and mitigate risks.
- Medical device QMS is required to effectively impart training programs to technical personnel and other staff members. Additionally, QMS insights can be used to customize staff training programs as per requirements.
- Medical device quality management system ensures smooth regulatory compliance.
- Quality management system for medical devices can be used to attain environmental and sustainability goals.
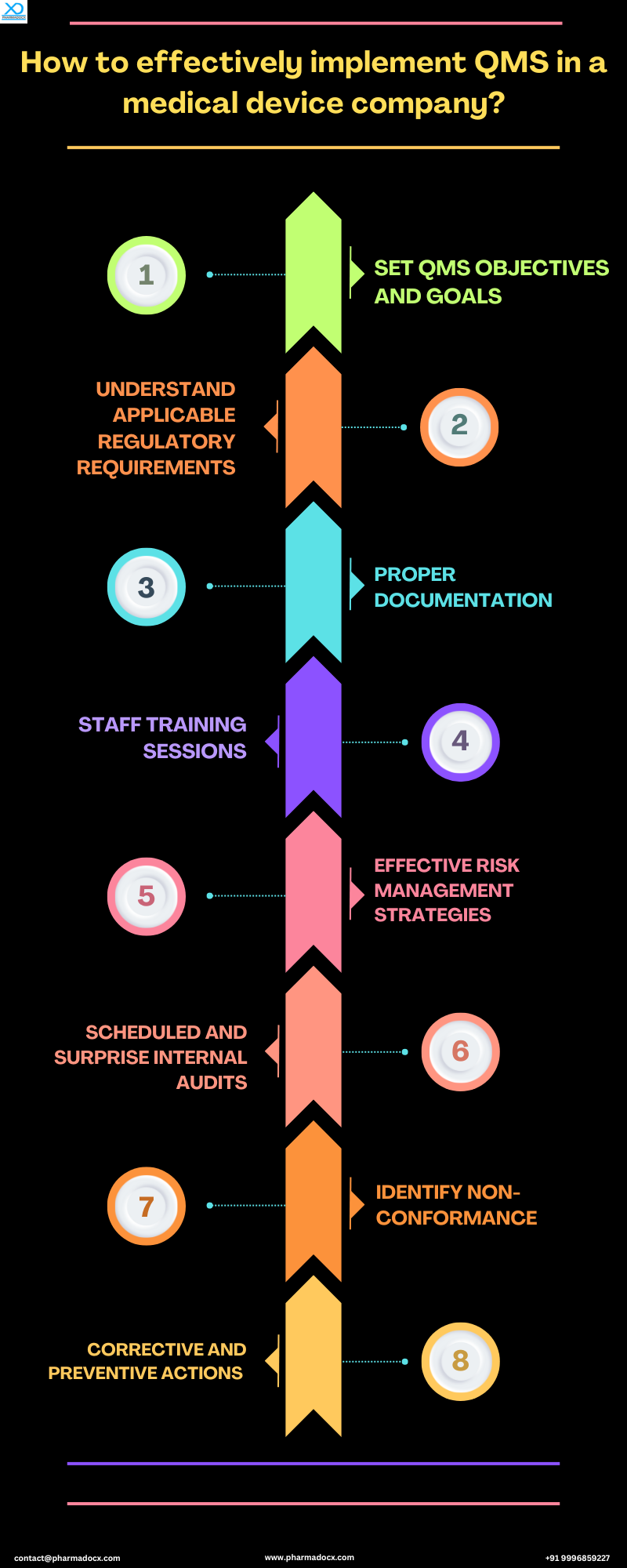
How to effectively implement medical device quality management system?
Implementing quality management system in a medical device company is vital for maintaining regulatory compliance and high product quality standards. Thus, we have provided a step-by-step guide for effectively implementing a robust quality management system for medical devices.
Understand applicable regulatory requirements
To ensure regulatory compliance in a medical device company, it is vital to understand the applicable regulatory requirements. You need to have a thorough knowledge of ISO 13485, FDA regulations, and other regulations for medical device quality management system. Furthermore, you need to identify any specific requirements applicable for your medical device. As a medical device company, it is not possible to be aware of all the nuances of the QMS industry regulations.
This is where Pharmadocx Consultants medical device QMS regulatory support comes into picture. Our team of regulatory experts has extensive knowledge of the regulatory requirements and will help you determine all the applicable regulations. Thus, we will leverage our expertise in QMS regulatory guidelines to help you achieve compliance.
Set quality objectives and medical device quality management system goals
Clearly define QMS objectives and policies. Establish medical device QMS objectives that align with the industry quality regulatory standards. Additionally, develop comprehensive medical device QMS policies. Clearly defining the objectives and policies will help you effectively implement QMS in your medical device company.
Effective risk management
A robust risk management system will help identify, assess, and mitigate risks associated with medical devices. A proactive and continuous approach is required to identify any quality lapses. Risk management is one of the pillars of quality management system for medical devices.
Proper documentation
All the processes involved in the medical device production should be properly documented for compliance and consistency. From raw material procurement to final product packaging, every detail must be meticulously documented. Proper documentation is the backbone of effective QMS. Proper documentation ensures traceability and facilitates audits. Additionally, it has an indispensable role in maintaining product quality and regulatory compliance.
Avail our QMS documentation service for in-depth documentation of your medical device manufacturing and quality control process. Our team will meticulously record every detail for easy access, audit, and traceability.
Staff and personnel training
It is vital to educate your team about the importance of regulatory compliance and effective quality control. Training session is essential for successful QMS implementation in a medical device company. Moreover, continuous and regular training will keep your personnel updated with the latest regulatory guidelines.
Our team of experts at Pharmadocx Consultants conducts staff training sessions and workshops for medical device companies. We will conduct training programs to educate your staff on how to effectively implement QMS in medical device company. Additionally, we will update them with the latest regulations and guidelines.
Manage non-conformances and implement CAPAs
Identify non-conformance and ensure timely resolution of identified lapses and issues. CAPAs stands for corrective and preventive actions. Under CAPA, corrective actions are implemented to address current issues and preventive actions are implemented to ensure they don’t reoccur. CAPA forms the basis of medical device quality management system.
Continuous improvement
Medical device QMS has to be consistently updated and modified per industry standards. Moreover, it is vital to regularly review your QMS strategies and policies. Also, it is crucial to foster an environment of continuous improvement and innovation that will improve the product quality. Moreover, this will lead to consistent regulatory compliance.
Continuous and surprise internal audits
Regular scheduled and surprise internal audits are vital for effectively implementing QMS in medical device companies. Moreover, the audit findings should be properly documented. Additionally, any corrective measures and actionable next steps should be determined.
Pharmadocx Consultants team conducts mock audits to help medical device companies identify any lapses. Our audit team will monitor and measure the performance of the QMS in your company. Furthermore, we not only identify lapses but also provide actionable next steps.
Why choose Pharmadocx Consultants?
Medical Device Licences
Years Experience
Plants Set-up
Our clients

Pharmadocx Consultants: Your trusted medical device quality management system support
Our comprehensive QMS support covers regulatory compliance, mock audit, documentation, staff training, and other major aspects of quality management system. By availing our medical device quality management system service, you can effectively implement QMS in your medical device company. Moreover, our medical device QMS support team will mentor and guide you. Furthermore, we have extensive knowledge and expertise in quality management and Indian regulations for medical devices.
Let's Talk!
We'd love to hear from you! Whether you have questions about our pharmaceutical plant setup consultation services or want to discuss a potential project, our team is here to help. Simply fill out the form below, and we'll get back to you as soon as possible. Alternatively, you can reach out to us directly using the phone number or email address listed on this page. We look forward to connecting with you!
Phone / Whatsapp
Address
- Head Office - Opposite Dewan Mill, Old D.C. Road Sonepat - 131001 Haryana, India
- Registered Office - Netaji Subhash Place, Delhi, 110034