US FDA Cosmetics Registration India MoCRA
The US laws for cosmetics have changed. They have become comprehensive and stringent with strong focus on consumer safety. To increase transparency and safety in the cosmetics industry, the Modernization of Cosmetics Regulation Act (MoCRA) has been implemented. By introducing new and strong regulations, MoCRA enhances FDA’s authority over the cosmetics industry. MoCRA implementation will impact cosmetic manufacturers, contract manufacturers, importers, packers, and distributors. Especially with the implementation of MoCRA, we understand the difficulties cosmetics companies face in navigating the US regulations. Avail our all-encompassing US FDA cosmetics regulatory service to be completely stress free. As a MoCRA US agent, Pharmadocx Consultants will assist you with all applicable cosmetics regulations. We provide tailored services to our client to help them comply with the US cosmetics regulatory guidelines.
Trusted MoCRA US agent: How can Pharmadocx Consultants make your product MoCRA-compliant?
We have a wealth of experience in MoCRA guidelines and US FDA cosmetics regulatory requirements. Our team of seasoned experts is dedicated to provide exceptional customer service.
We will assume full responsibility for achieving MoCRA compliance so that you can market your products in US without regulatory hiccups. As a professional MoCRA US agent, we adopt a customer-centric and efficient approach to help our clients achieve MoCRA compliance.
You can rest easy and focus on other aspects of establishing the cosmetics business, as we will take care of all regulatory requirements.
The Pharmadocx Consultants team will help you easily navigate the MoCRA regulations from facility registration to adverse event reporting. Our comprehensive US FDA cosmetics regulatory service is all you need to seamlessly comply with all MoCRA guidelines.
Is a MoCRA US agent required for cosmetics facility registration?
Yes, a MoCRA US agent will be required for cosmetics facility registration, if the company is located outside of US. The MoCRA US FDA agent will act on behalf of the foreign cosmetics manufacturer to register the facility with the FDA as per MoCRA requirements. Notably, the agent has to be authorized by the foreign manufacturer to act on their behalf.
Moreover, hiring a professional MoCRA US agent has many advantages. Firstly, the agent knows the MoCRA requirements in details and will help you complete the cosmetics facility registration correctly. Secondly, chances of getting fines and penalties are low when the support of an agent is used. Finally, you do not need to get into the nitty-gritties of MoCRA guidelines, as the agent will take care of the requirements on your behalf.
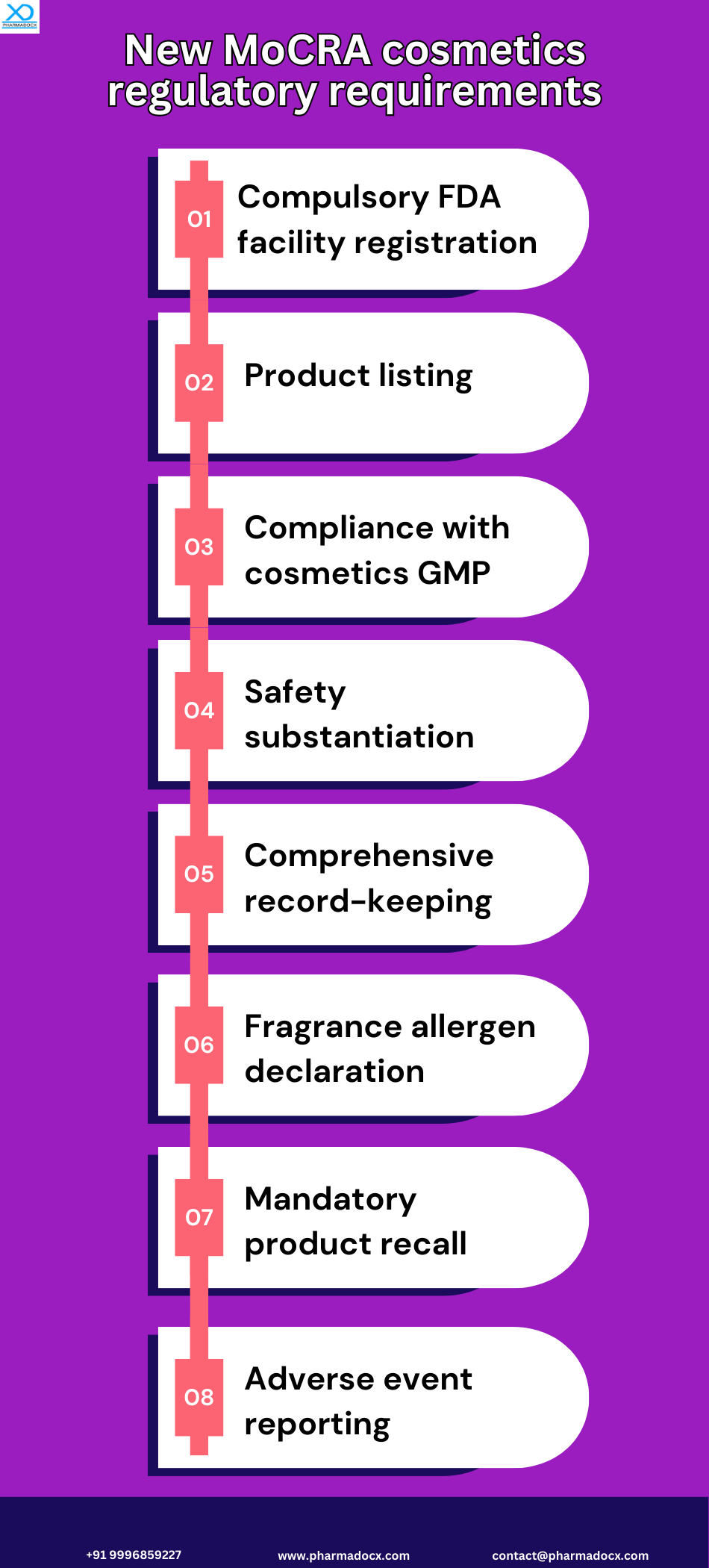
How to make your cosmetics product MoCRA-compliant?
Focussing on consumer safety, MoCRA has laid down certain guidelines that cosmetics manufacturers will have to abide by. Notably, these guidelines aim to increase transparency and cosmetics manufacturer’s accountability. You need to adhere to MoCRA requirements to make your cosmetics products MoCRA-compliant. As a trusted MoCRA US agent, we can help you achieve MoCRA compliance. We have presented the primary MoCRA requirements.
Cosmetics facility
registration
All cosmetics facilities will have to be registered with the US FDA. Furthermore, the facility registration has to be renewed every 2 years.
Cosmetics product listing
The responsible person will be required to annually update each marketed cosmetic product with the FDA. Moreover, they are also required to provide information on product ingredients.
Safety substantiation
MoCRA mandates the responsible person to maintain records proving product safety and efficacy. The records can contain results from tests and studies and other expert-approved evidence.
Cosmetics labelling requirements
MoCRA has introduced new cosmetics labelling requirements. Contact information for reporting adverse effects, fragrance allergen disclosure, and professional use product labelling are some of the major requirements.
Cosmetics good manufacturing practices
MoCRA guidelines require cosmetics facilities to abide by GMP guidelines. Furthermore, this regulation allows the US FDA to inspect facilities and access necessary records to verify GMP compliance.
Adverse events reporting
MoCRA guidelines mandate the responsible person to promptly report any serious adverse within 15 business days. Additionally, companies will be required to retain adverse event records for the cosmetic product usage for 6 years (3 for small businesses).
Pharmadocx Consultants: We provide comprehensive US FDA cosmetics regulatory service
Our aim is to help cosmetics companies launch their products in US without regulatory hurdles. We provide support as a MoCRA US agent. Our team will assist you comply with MoCRA guidelines, by:
- Reviewing your product labels, technical documents, and safety records
- Additionally, we will help you register your facility, list your product, and report adverse events
Moreover, the cosmetics regulatory landscape is ever evolving and dynamic. Hence, we will keep you updated with the latest changes in cosmetics regulation and help you comply with them.
Do you want to check whether your product is MoCRA complaint?
Get in touch with us
Common FAQs every MoCRA US agent is asked
What is MoCRA?
MoCRA stands for Modernization of Cosmetics Regulation Act of 2022. It aims to strengthen the regulatory oversight for cosmetic products, facilities, and companies
Who is the MoCRA responsible person?
The MoCRA responsible person is the manufacturer, packer, or distributor of a cosmetic product whose name appears on the product label in accordance with section 609(a) of the FD&C Act or section 4(a) of the Fair Packaging and Labeling Act. The duties of the MoCRA responsible person are cosmetic product listing, adverse event reporting, safety substantiation, correct product labelling, and facility registration.
What is the MoCRA product listing requirements?
As per MoCRA guidelines, each marketed cosmetic product, including its ingredients, has to be listed with the US FDA within 1 year of MoCRA’s enactment. Furthermore, for products marketed after MoCRA’s enactment, a complete listing has to be submitted to the FDA within 120 days of marketing. Additionally, the responsible person must annually update the product listing information.
What is the difference between cosmetics facility registration and cosmetics product listing?
As per MoCRA guidelines, for cosmetics facility registration, companies are required to register their facilities with the FDA. Additionally, they must renew their facility registration every two years. As per MoCRA guidelines, for cosmetics product listing, the responsible person must list each marketed cosmetic product with the FDA. Additionally, the listing has to be updated annually.
How will I list multiple cosmetics product categories under MoCRA?
MoCRA has the option for flexible listings using a flexible submission form for cosmetics products with minor variations. In case of flexible listings, a single listing for cosmetic products with identical formulations or formulations with slight variations can be submitted. The products can differ only with respect to fragrances, flavours, quantity of contents, or colours.
What is the MoCRA mandatory product recall?
MoCRA has bestowed the FDA with legal authority to order a mandatory recall for products that they consider adulterated or misbranded. If the authorities feel exposure to a cosmetics product may cause serious adverse health effects, they may demand a recall. Once a mandatory recall is issued, the responsible person will be obliged to recall the products.
Who is exempt from MoCRA requirements?
Small businesses whose average gross annual cosmetic products sale for the previous 3 years is less than USD 1 Million are exempt from MoCRA requirements. They are exempt from MoCRA requirements, such as facility registration, GMP, and product listing requirements. To be eligible for the exemption, the business should not be involved in manufacturing/processing cosmetic products that can be injected. Additionally, the products should not alter appearance for more than 24 hours without consumer removal. Furthermore, they should not be intended for internal use. Moreover, they should not come into contact with the eye’s mucus membrane.
What is the need for the cosmetics good manufacturing practices under MoCRA guidelines?
MoCRA guidelines mandate cosmetics facilities to adhere to cosmetics good manufacturing practices (GMP). This will align the cosmetics manufacturing process with international standards. Hence, high-quality and safe cosmetics products will be consistently manufactured. Therefore, adulterated and substandard products will not enter the US cosmetics market, thereby protecting consumer health.
What are the new cosmetics labelling requirements introduced by MoCRA?
MoCRA guidelines have introduced certain new mandatory labelling requirements for cosmetics products. Firstly, the domestic address, domestic phone number, or electronic contact information of the person responsible for receiving adverse event reports has to be included. Additionally, fragrance allergens present in the product have to appropriately flagged. Furthermore, cosmetics intended for use by professionals should be properly labelled as “professional use” products.
Let's Talk!
We'd love to hear from you! Whether you have questions about our pharmaceutical plant setup consultation services or want to discuss a potential project, our team is here to help. Simply fill out the form below, and we'll get back to you as soon as possible. Alternatively, you can reach out to us directly using the phone number or email address listed on this page. We look forward to connecting with you!
Phone / Whatsapp
Address
- Head Office - Opposite Dewan Mill, Old D.C. Road Sonepat - 131001 Haryana, India
- Registered Office - Netaji Subhash Place, Delhi, 110034
