Your Guide to New MoCRA Cosmetics Labelling Requirements
Implementation of the Modernization of Cosmetics Regulation Act (MoCRA) is a landmark moment in the cosmetics product regulatory history. By enforcing new regulatory requirements, it represents a significant shift from the previous cosmetics regulatory guidelines. Among the various requirements it has introduced, MoCRA aims to strengthen the regulations for labelling cosmetics products. These regulations have been introduced to ensure transparency and improve consumer safety. Implementation of MoCRA has significantly transitioned the cosmetics regulatory guidelines. Hence, FDA authorities are strictly implementing cosmetics labelling guidelines. Thus, cosmetics businesses need to be aware of these new guidelines. Additionally, they should update their product label to comply with the new MoCRA cosmetics labelling requirements. However, these requirements are elaborate and intricate. Hence, we have created this guide to help you understand the new MoCRA guidelines for labelling cosmetics products.
Furthermore, our experts can help you meet MoCRA cosmetics labelling requirements. Cosmetics product label will have to comply with various guidelines, especially fragrance allergen disclosure and adverse event reporting contact information. We can check your cosmetics product label for MoCRA compliance. Our team will not only flag instance of non-compliance but also provide detailed recommendations to help you achieve MoCRA compliance.
What are the new MoCRA cosmetics labelling requirements?
The MoCRA guidelines have introduced new labelling requirements for cosmetics products. Understanding the changes in cosmetics labelling requirements is crucial for MoCRA compliance. Hence, we have highlighted the key changes in cosmetics labelling requirements introduced by MoCRA.
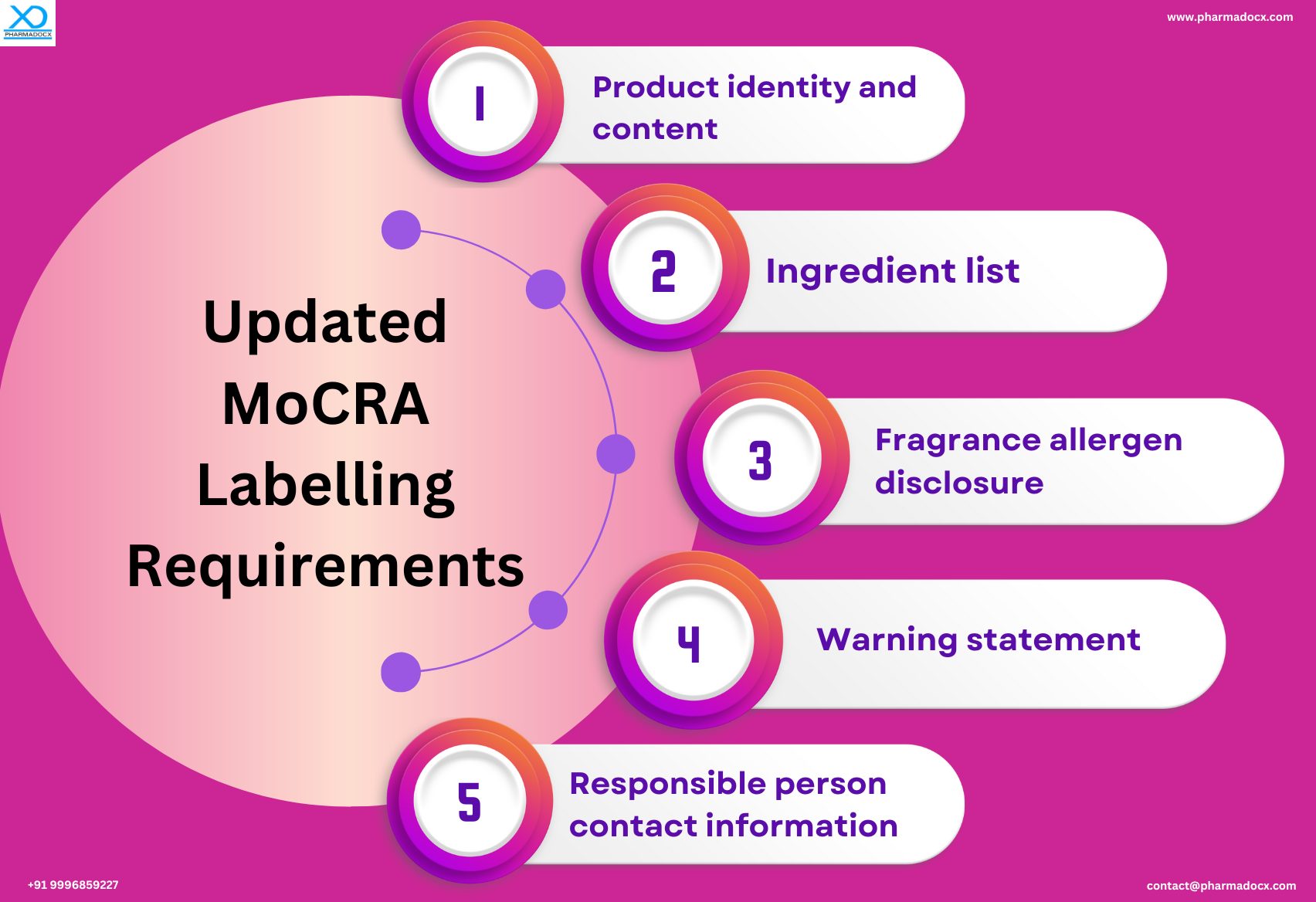
Product identity statement and net contents
The cosmetics product’s identity, such as “shampoo” or “moisturizer,” should be clearly mentioned on the cosmetics product label. Additionally, the amount of product in the package, expressed in weight or numerical count, should be mentioned on the label.
Disclosure of ingredients
Every ingredient used in the cosmetic product should be clearly listed on the label. The product ingredients have to be listed in descending order of predominance as per their weight. The ingredient components that directly affect the skin or hair have to be mentioned. Additionally, other components, including those ingredients that contribute to the product’s fragrance, texture, or preservation, have to be listed.
Allergen disclosure
MoCRA guidelines mandatorily require manufacturers to disclose the presence of any potential allergens in their products. Common allergens, such as fragrance allergens, certain preservatives, and plant extracts, are usually present in the cosmetics products. Fragrance allergen disclosure is a very important requirement of MoCRA guidelines for cosmetics product label.
Cautionary warnings and directions for use
MoCRA guidelines place special emphasis on consumer safety and health. Hence, proper instructions on how to safely use a cosmetic product is required to ensure it does not harm the consumer. Thus, MoCRA cosmetics labelling requirements mandate clear warnings and instructions for use to be included on the cosmetics product label. Warnings on potential risks associated with product usage and how to avoid them should be mentioned. Additionally, clear and concise directions on how to safely use the cosmetics product have to be mentioned. Moreover, the cautionary warnings and/or directions for safe use should be presented in a noticeable manner on the label.
Product expiration dates
The FDA guidelines do not exactly require the product label to mention the expiration date. However, the manufacturer is accountable for product safety and any adverse effect caused by the product. Hence, to be safe the manufacturer should mention the product expiration date on the label. By mentioning the period within which the product is safe and effective, the manufacturer can ensure consumer safety.

Responsible person contact information
The responsible person is required to receive adverse events reported by consumers. Hence, the MoCRA guidelines require the cosmetics product label to mention the domestic phone number, address, or electronic contact information of the responsible person. The responsible person can be the manufacturer, packer, or distributor of the cosmetics product whose contact information should be displayed on the label. The company name, US address, US Telephone number, or electronic contact need to be mentioned on the label. This MoCRA guideline is intended to facilitate consumer inquiries and an adverse event contact person support.
Importance of compliance with MoCRA cosmetics labelling requirements
MoCRA guidelines have placed special focus on consumer safety. The MoCRA cosmetics labelling requirements align with this goal. Manufacturers are mandatorily required to comply with these MoCRA guidelines. Moreover, they are expected to update their existing product labels to ensure they comply with the latest guidelines. Non-compliance with guidelines for labelling cosmetics products can lead to legal and regulatory consequences. Manufacturers can avoid the following negative consequences by strictly following labelling guidelines:
- Mandatory product recalls: Failure to comply with cosmetics labelling guidelines can be interpreted as misbranding or incorrect labelling of products. This can lead to mandatory product recalls.
- Sanctions, fines, and penalties: Cosmetics companies can be slapped heavy fines and legal penalties for not complying with the labelling requirements.
- Damage to brand name and reputation: Consumers usually prefer cosmetics brands that demonstrate regulatory compliance. Non-compliance with MoCRA guidelines can harm the company’s brand name and reputation. Additionally, it will hurt consumer trust in the brand.
Hence, compliance with MoCRA labelling requirements is vital.
Not sure whether your product label complies with MoCRA guidelines?
Get in touch with our team of experts for guidance
How to ensure compliance with MoCRA cosmetics labelling requirements?
Compliance with MoCRA cosmetics labelling requirements is of paramount importance. However, the labelling guidelines have various parameters and requirements. Hence, we have provided some tips to help you achieve MoCRA compliance.
- Review the MoCRA product label guidelines: MoCRA has laid down detailed guidelines for labelling cosmetics product. Notably, the guidelines focus not only on the content but also on label size and placement. Moreover, there are guidelines for fragrance allergen disclosure, adverse even report contact person information, disclosure of ingredients, and warning statements. Hence, it is vital to thoroughly review the cosmetics product labelling guidelines before proceeding with updating the labels.
- Product label: Check the product label size, placement, type, and prominence requirements for your product. Ensure your product label is large enough that the required information will be prominently displayed in a type size that is easily legible.
- Audit product ingredients: Perform an audit of all the ingredients present in the cosmetics product to ensure they are correctly listed on the label.
- Review all existing product labels: Thoroughly review current product labels to identify areas of non-compliance. Refer to the product label guidelines to verify whether your product labels need to modified.
- Update product labels: Update your labels to ensure they display all the required information clearly as per MoCRA guidelines.
- Staff training and workshop: Impart thorough training on cosmetics product labelling requirements. Ensure your staff is aware of the latest guidelines and requirements. Every member of the team should understand the importance of compliance.
- Regular compliance audit: Regularly conduct internal audits to ensure compliance with latest MoCRA guidelines and regulations.
Pharmadocx Consultants: Your trusted guide for MoCRA cosmetics labelling requirements
MoCRA has transitioned the regulatory landscape for cosmetics products in the US. It has introduced new requirements and guidelines for cosmetics products marketed in the US. Among the various requirements introduced, the updated cosmetics labelling guidelines is one of the vital ones. Hence, by complying with the new MoCRA cosmetics labelling requirements, companies can avoid regulatory sanctions for incorrect product labelling. Thus, it is vital to proactively update your cosmetics product labels to ensure MoCRA compliance. Contact Pharmadocx Consultants’ team of experts to ensure holistic compliance with MoCRA guidelines for cosmetics products.
Let's Talk!
We'd love to hear from you! Whether you have questions about our pharmaceutical plant setup consultation services or want to discuss a potential project, our team is here to help. Simply fill out the form below, and we'll get back to you as soon as possible. Alternatively, you can reach out to us directly using the phone number or email address listed on this page. We look forward to connecting with you!
Phone / Whatsapp
Address
- Head Office - Opposite Dewan Mill, Old D.C. Road Sonepat - 131001 Haryana, India
- Registered Office - Netaji Subhash Place, Delhi, 110034
