Saudi Arabia SFDA Medical Device Registration Consultant
Medical devices are in high demand in the Kingdom of Saudi Arabia. The Saudi Food and Drug Authority (SFDA) regulates all medical devices and IVDs being sold and distributed in Saudi Arabia. Hence, to market medical devices in Saudi Arabia, a medical device market authorization (MDMA) from the SFDA is required. The SFDA medical device registration process has extensive guidelines, documentation requirements, and rigorous quality standards. Moreover, recently, Saudi Arabia has updated their medical device regulations. Hence, suppliers of even previously approved medical devices are expected to comply with the new Saudi Arabia medical device regulations. Thus, securing the medical device registration in Saudi Arabia is cumbersome and requires knowledge of the latest regulations. A Saudi Arabia medical device registration consultant can make this mammoth task a cake walk for you. We at Pharmadocx Consultants can help you have a smooth SFDA medical device registration process.
Consultant for Saudi Arabia Medical Device Registration
The Saudi Arabia medical device regulations are dynamic and are constantly updated to protect patient health. As a medical device manufacturer, it is not to stay abreast of the latest Saudi Arabia medical device regulatory guidelines.
Assistance from a medical device registration consultant can make the process of medical device registration in Saudi Arabia easy for you. The consultants have an extensive knowledge of the medical device regulatory landscape and have expertise in the SFDA medical device registration process. The Saudi Arabia medical device registration consultants can assist manufacturers in complying with the necessary regulatory requirements, documentation requirements, and quality standards essential for obtaining SFDA approval.
Hence, these consultants can help manufacturers easily launch and sell their medical devices in the Saudi Arabian market.
Thus, a SFDA medical device registration consultant can help you efficiently navigate regulatory complexities and increase the chance of SFDA approval.
Need help with medical device registration in Saudi Arabia?
Fill out the form below and we will get in touch
Saudi Arabia Medical Device Classification
The Saudi Arabia medical device registration process varies depending on the medical device class. Hence, it is important to understand Saudi Arabia medical device classification system. The SFDA has classified medical devices into four classes based on their risk level. Notably, grouping of medical device models, variants, accessories into a single application is permitted per SFDA medical device regulations.
- Class A: Low risk medical devices. Class A has some sub categories. Low to medium risk sterile medical devices, measuring medical devices, and reusable surgical instruments form a part of Class A medical device subcategory.
- Class B: Low to medium risk medical devices
- Class C: Medium to high-risk medical devices
- Class D: High risk medical devices
Documents required for Saudi Arabia medical device registration
We have provided an overview of the documents required.
1. Manufacturer and the Saudi authorized representative details.
2. Device information (including accessories): Trade name in English and Arabic (if the device is intended for lay use) and model name(s)/number(s), catalogue number(s), etc. have to be provided. Additionally, device description and intended purpose have to be mentioned. The medical device classification has to be mentioned. Evidence of approval in other countries, if available, may be provided.
3. Device label: Device labels, packaging, and power supply label, where applicable, have to be provided. Additionally, instructions for use (IFU) or justification letter, if not available, have to be included. Storage, transportation, installation, maintenance, and disposal information has to be mentioned. Furthermore, barcodes and proposed advertising materials are to be provided.
4. Design and manufacturing information: Description of function, medical device design, technical specifications, bill of materials, and assembly of the device have to be provided. Additionally, manufacturing process flow has to be included.
5. Essential principles checklist
6. Benefit-risk analysis and risk management: Risk management plan, risk analysis, and risk management report have to be provided.
7. Product verification and validation: Pre-clinical data and clinical data have to be mentioned.
8. Post-market surveillance plan
9. Periodic safety update report
10. A statement certifying that the applicant will follow the National Centre of Medical Devices Reporting (NCMDR) requirements has to be provided.
We have listed the documents and information that must be included in the technical documentation file required for the Saudi Arabia medical device registration process. This is only an overview of the documents required. For a detailed list of documents required, feel free to reach out to us. Additionally, our team will help you prepare and compile the documents as per regulatory guidelines.
Saudi Arabia Medical Device Registration Process
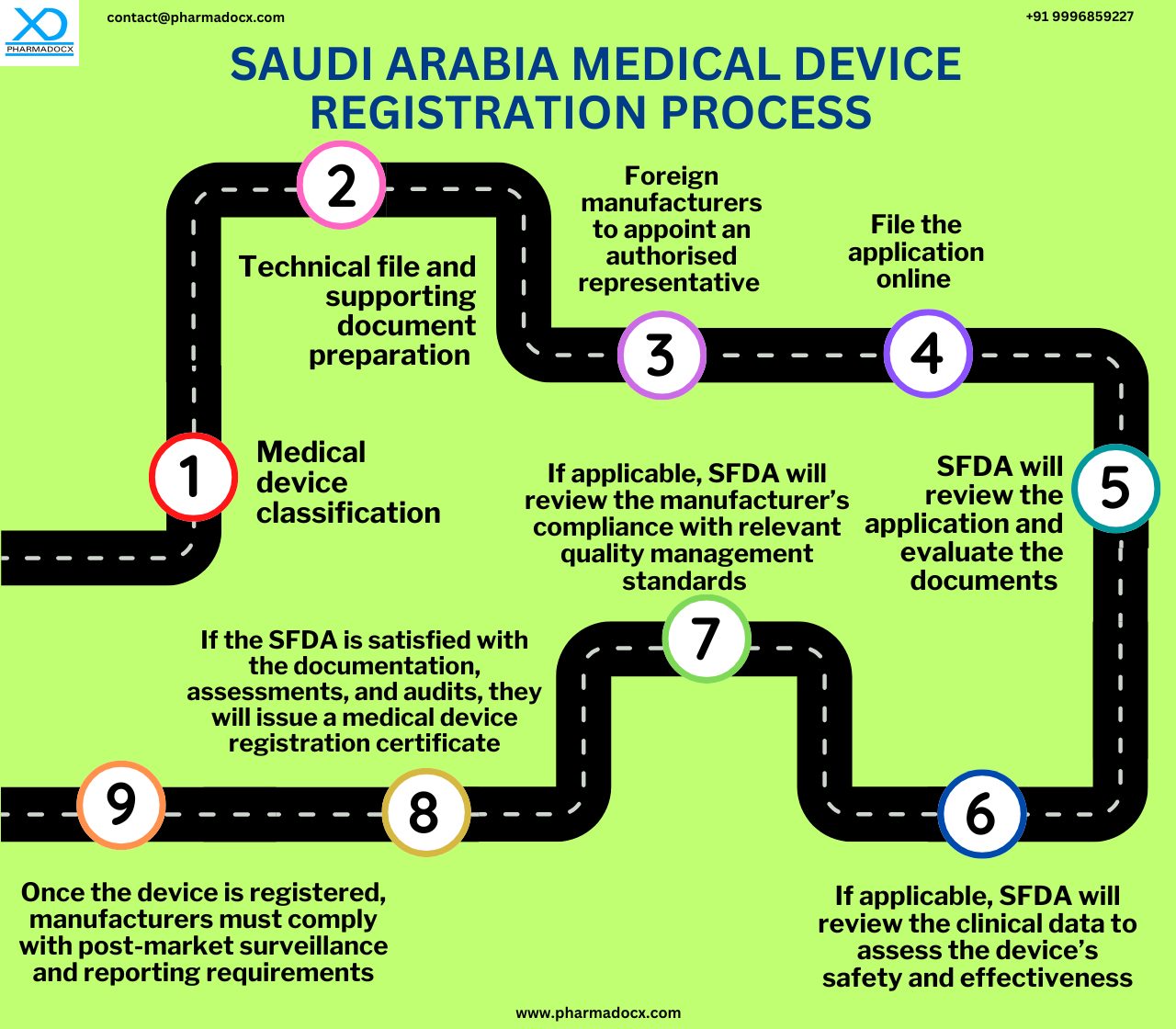
The Saudi Arabia medical device registration process pathway will depend on the medical device class. There are two primary SFDA medical device registration pathways.
Medical Device National Registry (MDNR) Listing
For non-sterile/non-measuring low risk medical devices, listing in the Medical Device National Registry (MDNR) is required as a pre-requisite for marketing the device in Saudi Arabia. This listing can be done by any establishment importing or distributing the device in the country. Basic product and manufacturer information, proof of QMS, and reference country approval are required. Additionally, device labelling and marketing materials are also required. The SFDA usually grants approval in 4 working days. Furthermore, this approval is valid for 3 years.
Medical Device Marketing Authorization (MDMA)
For all other classes of devices, medical device approval issued as Medical Device Marketing Authorization (MDMA) is required to market the device in Saudi Arabia. The SFDA medical device registration timeline for the MDMA approval is usually 35 days. Furthermore, the license will remain valid for the period of original license validity or 3 years for undefined original license validity.
Saudi Arabia Authorized Representative
All foreign manufacturers not having a legal entity or physical presence in Saudi Arabia are required to appoint a medical device authorized representative (AR). The AR will act on behalf of the manufacturer in Saudi Arabia. Notably, the AR must have an Authorized Representative License issued via the medical device establishment (MDEL) system. The AR will be responsible for submitting the application to the SFDA to register your medical device.
Saudi Arabia Medical Device Registration Process: What are the Steps?
Our Clients
Pharmadocx Consultants: Trusted SFDA Medical Device Registration Consultant
We possess in-depth knowledge of the Saudi Arabia medical device regulations. As a trusted Saudi Arabia medical device registration consultant, we offer comprehensive regulatory service. We will help identify the correct medical device class, prepare documents, file the application, prepare query response and provide assistance till you successfully secure your registration. Moreover, our support does not end with successful SFDA medical device registration. We also provide post license approval support and guidance. Our aim is help you secure swift and efficient SFDA approval. We tailor our services per client needs. Drop an email at [email protected] or call/Whatsapp on 9996859227 for seamless medical device registration in Saudi Arabia.
Let's Talk!
We'd love to hear from you! Whether you have questions about our pharmaceutical plant setup consultation services or want to discuss a potential project, our team is here to help. Simply fill out the form below, and we'll get back to you as soon as possible. Alternatively, you can reach out to us directly using the phone number or email address listed on this page. We look forward to connecting with you!
Phone / Whatsapp
Address
- Head Office - Opposite Dewan Mill, Old D.C. Road Sonepat - 131001 Haryana, India
- Registered Office - Netaji Subhash Place, Delhi, 110034

