US FDA Medical Device Labelling Guidelines: Your Guide
Compliance with US FDA medical device labelling requirements is key to get your device to the US market. Informative and accurate medical device labelling is required. Every medical device label included with the device has to meet the applicable standards and regulations. The label is used to communicate operation, storage, risks, and intended use of the device to users. Proper medical device labelling will ensure the users safely and effectively receive benefits of the device. Whereas, improper labelling can result in harm to the user from usage of the device. Medical device labelling is a crucial part of the production process for medical device manufacturers. Moreover, medical device regulations cover everything from label design to how they are affixed to the device. Hence, devices cannot be sold in the market without the required labels.
What is a Medical Device Label?
The US FDA has provided a clear definition for a medical device label. A medical device label is a display of written, printed, or graphic matter on the device and any of its containers or wrappers. Additionally, the definition covers packaging labels, instructions for use (IFU), user interface screens or embedded software messaging, product inserts and manuals, and promotional or training materials accompanying the device. It also covers direction sheets, posters, circulars, booklets, tags, pamphlets, brochures, fillers, etc. accompanying the device.
The US FDA Medical Device Labelling Guidelines
Medical device labelling guidelines are mentioned in the following Parts of Title 21 of the Code of Federal Regulations (CFR).
- General device labelling: 21 CFR Part 801
- Use of symbols: 21 CFR Part 801.15
- In vitro diagnostic products: 21 CFR Part 809
- Investigational device exemptions: 21 CFR Part 812
- Unique device identification: 21 CFR Part 830
- Good manufacturing practices: 21 CFR Part 820
- General electronic products: 21 CFR Part 1010
Why is Proper Medical Device Labelling Important?
Patients, medical professionals, and experienced users require clear instructions for use, guidance, and warnings to safely and effectively use the medical device. Moreover, many medical devices are designed for use by patients in their homes without direct supervision from a medical professional. These devices specially require proper labelling. Improper or incorrect medical device labelling can lead to misuse or incorrect use of the device, thereby leading to the device causing harm to the user. This can negatively impact patient outcomes or can even cause serious injury or death. Hence, medical device labels are more than just stickers. They are required for safe and effective healthcare delivery, by ensuring correct usage of the medical devices.
- Patient safety: Medical device labels provide clear instructions to healthcare professionals and patients. This is vital for correct device usage, minimizing risks, and ensuring patient safety.
- Risk management: Proper medical device labelling helps reduce potential hazards associated with device misuse.
- Regulatory compliance: Adherence to US FDA medical device labelling guidelines is mandatory to legally market your device in US.
- Market accessibility: Compliance with medical device labelling guidelines increases market access.
Need help with US FDA labelling guidelines?
Fill out the form with your query
US FDA Medical Device Labelling Requirements
The US FDA medical device labelling guidelines have formulated certain requirements that medical device labels should abide by. The device label should provide sufficient information to ensure the device is safely and effectively used. We have highlighted the basic requirements every medical label should meet:
- Device name and intended use: The medical device label should clearly state what the device is and its purpose.
- Manufacturer contact information: The manufacturer name, address, and contact details should be stated on the label.
- Indications, contraindications, and precautions: Who should use the device, who should not, and what risks may apply have to be stated.
- Instructions for use (IFU): The label should provide step-by-step usage directions, including setup, maintenance, and disposal requirements.
- Lot or serial number: The product serial or lot number should be clearly mentioned on the label. It helps with traceability in the event of product recall.
- Warnings and cautions: Labels should clearly indicate situations where incorrect use may cause harm. It should clearly mention all warnings and caution.
- Manufacture and expiration dates: The manufacture and expiration dates should be clearly mentioned on the label. This is especially critical for sterile or time-sensitive products.
- Unique device identifier (UDI): A mandatory alphanumeric code that identifies the medical device’s DI and PI components should be mentioned on the label. For full technical specifications, refer to the FDA UDI labelling requirements (21 CFR Part 830).
- Standard symbols: ISO 15223-1 symbols may be used on the label instead of text when accompanied by a glossary or reference. If symbols are not well known, a glossary or explanatory insert must be provided with the device as per FDA guidelines.
Furthermore, device labels should be accurate, not misleading, and prominently displayed. Additionally, they must be resistant to wear and tear, ensuring legibility throughout storage, distribution, and use.
Specific Guidelines for Devices Intended for Patient Use
US FDA medical device labelling guidelines have provided specifications for certain devices intended mainly for layperson or home use. The medical device labels should:
- Be written in plain and non-technical language that is easy to understand for layperson
- Use high-contrast and large font sizes so that the label is easily legible
- Clearly mention risks and precautions to be taken
- Include contact information for support or emergency situations
- Include visual aids or pictograms for key steps
Furthermore, the FDA encourages usability testing to confirm that patients can understand and follow the labelling without supervision. This is especially important for devices to be used by children, elderly, or patients with limited English proficiency. Moreover, the labels should be prominently displayed.
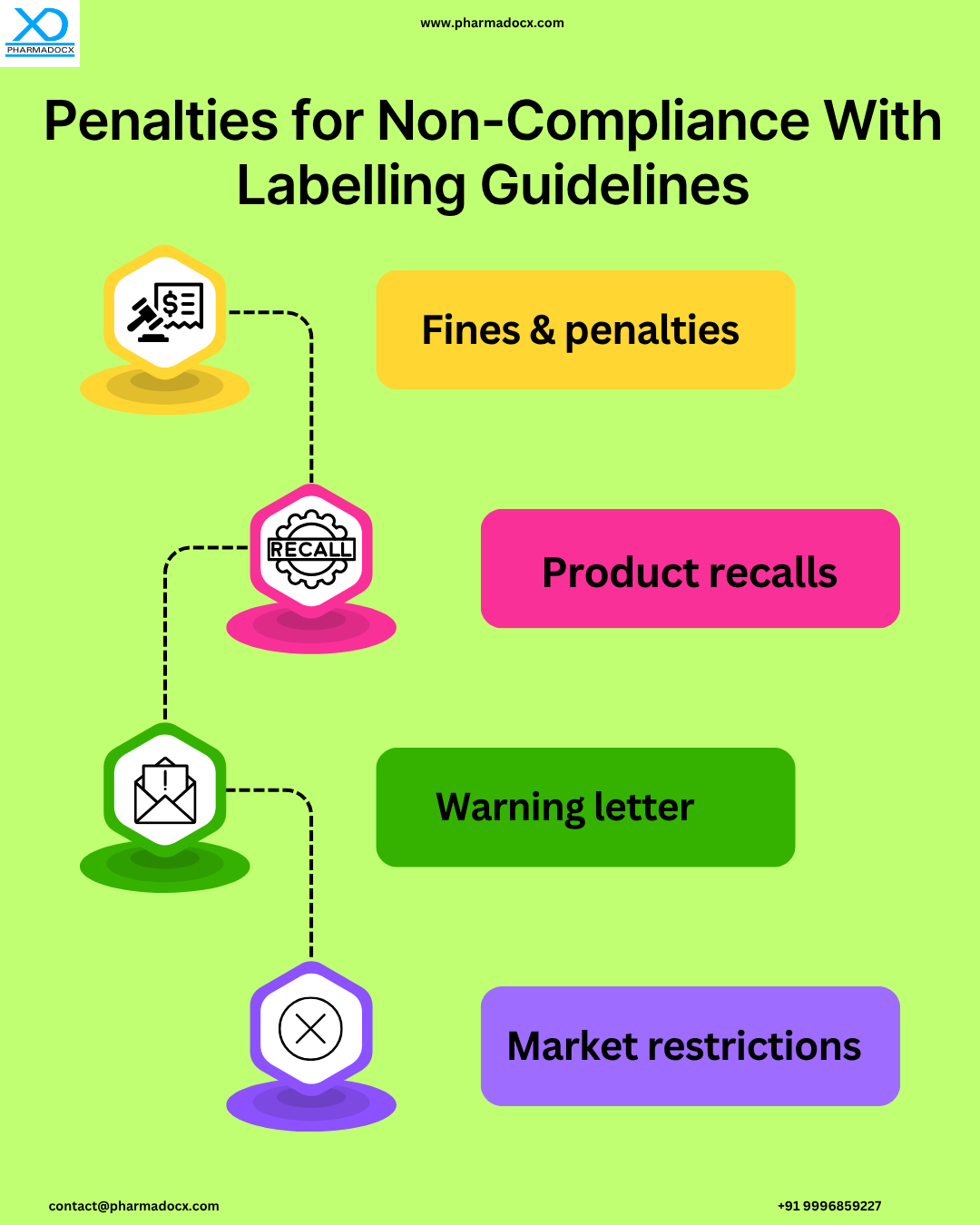
US FDA Medical Device Labelling Guidelines: General Medical Device Labelling Provisions
The US FDA medical device labelling guidelines mention the general medical device labelling provisions under 21 CFR Part 801. These guidelines outline the essential requirements for medical device labelling to ensure clarity, accuracy, and compliance. We have provided a brief overview of the provisions:
- Device manufacturer identification: The label should clearly display the name and place of business of the manufacturer, packer, or distributor.
- Directions for use: Instructions for use should be clearly mentioned. It should be adequate and clear to both healthcare professionals and laypersons to understand proper usage of the device.
- Business name requirements: If the device is manufactured by a company different from the one distributing it, the label must clearly specify the relationship (e.g., “Manufactured for ___” or “Distributed by ___”).
- Address details: The device labels are expected to include the street address, city, state, and ZIP code of the business, unless the address is available in a public directory.
- Unique device identifier (UDI): The device label should mention the UDI to facilitate tracking and identification.
- Legibility and placement: The label must be clear, prominent, and appropriately placed for easy readability.
Notably, the FDA conducts routine inspections to ensure the label meets US FDA medical device labelling guidelines.
6 Common FDA Medical Device Labelling Mistakes to Avoid
We have mentioned some of the common FDA medical device labelling mistakes. Additionally, we have provided some tips on how to avoid them.
- Unreadable or incomplete labelling: Medical device labels must be legible, complete, and accessible to users.
- Poorly structured instructions: The labels should have clear instructions, providing step-by-step guidance to prevent user errors.
- Contradictory information: Labels must align with the intended use of the device. Conflicting claims can result in regulatory action.
- Incorrect use of certification logos: Labels should not misuse certifying body logos, unless properly authorized.
- Post-clearance label alterations: Changing medical device labels post FDA approval without proper documentation will be considered compliance violation.
- Misplaced warnings and cautions: Safety warnings should be prominently displayed on the medical device label near relevant instructions.
We have extensive knowledge of the US FDA medical device labelling guidelines. We will thoroughly review your medical device label to ensure compliance with all applicable guidelines. For any US FDA regulatory support, feel free to email at [email protected] or call/Whatsapp on 9996859227.
Let's Talk!
We'd love to hear from you! Whether you have questions about our pharmaceutical plant setup consultation services or want to discuss a potential project, our team is here to help. Simply fill out the form below, and we'll get back to you as soon as possible. Alternatively, you can reach out to us directly using the phone number or email address listed on this page. We look forward to connecting with you!
Phone / Whatsapp
Address
- Head Office - Opposite Dewan Mill, Old D.C. Road Sonepat - 131001 Haryana, India
- Registered Office - Netaji Subhash Place, Delhi, 110034

