US FDA Medical Devices Establishment Registration
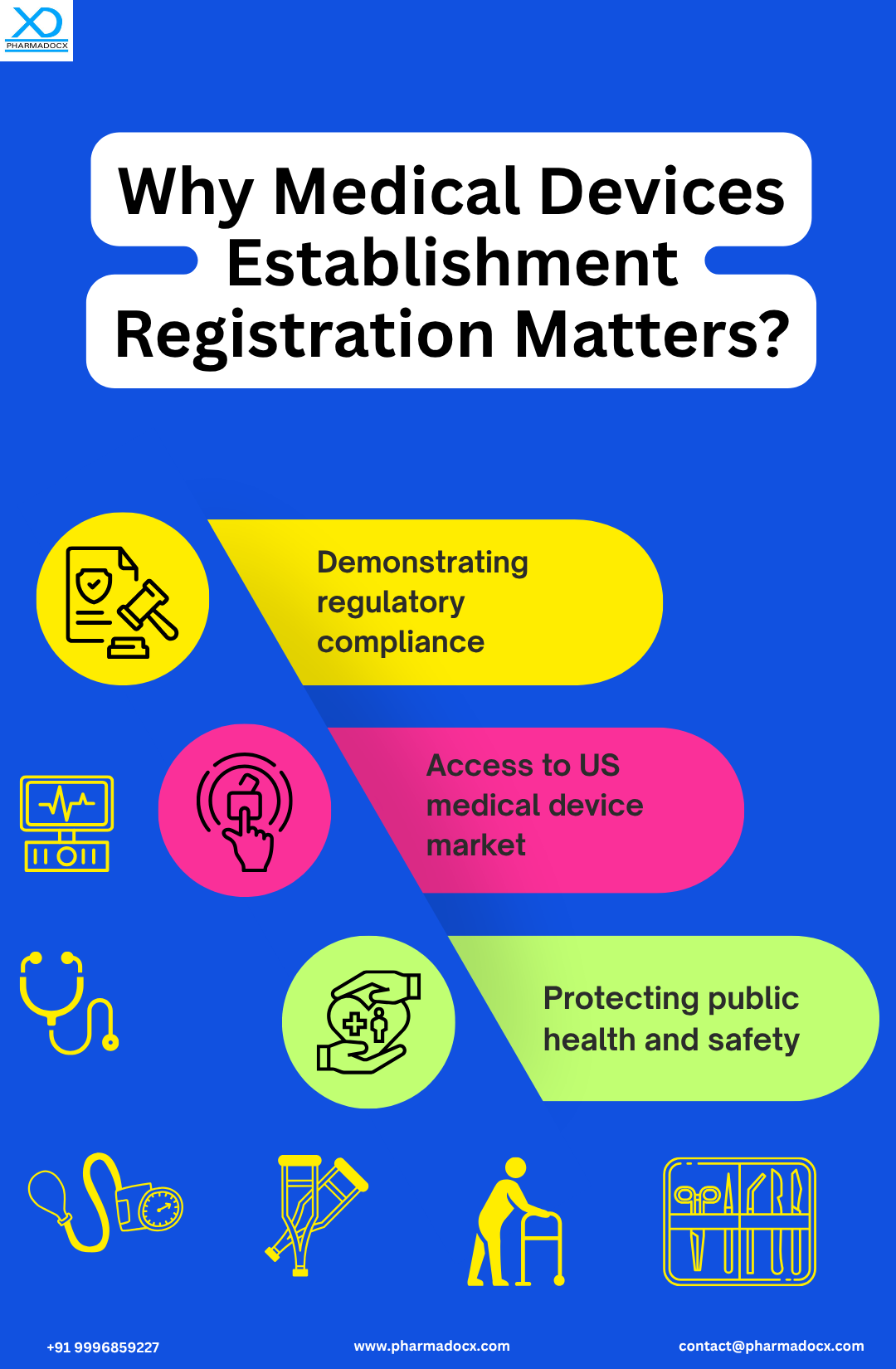
Medical devices entering the US market are strictly regulated to ensure patient safety and deliver improved healthcare. The US FDA medical devices establishment registration is an annual process mandated by the U.S. FDA. By implementing mandatory registration, the FDA ensures all medical device establishments abide by the established safety and performance standards. This requirement is vital for FDA to understand the public health preparedness. It acts as an early detection mechanism for issues within the supply chain and provides better oversight. Moreover, it facilitates timely responses in situations, such as recalls or safety alerts. Furthermore, registering your medical device establishment with the US FDA provides uninterrupted access to the U.S. market. Establishment registration is at the core of FDA’s regulatory framework. By registering the establishment, medical device companies can demonstrate regulatory compliance. We will help you easily comply with US FDA medical devices establishment registration and device listing requirements.
What is US FDA Medical Devices Establishment Registration?
US FDA medical devices establishment registration is the process of owners or operators of establishments involved in the production and distribution of medical devices registering with Food and Drug Administration (FDA). These entities involved in the production and distribution of medical devices intended for use in the U.S. must annually register with the FDA prior to selling their devices. As per Title 21 CFR Part 807, US FDA medical devices establishment registration is a mandatory requirement. Notably, registration must be done electronically on the FDA Unified Registration and Listing System (FURLS system). Moreover, annual verification of registration details is a mandatory obligation. The verification can be completed at any time between the 1st of October and 31st of December each year.
The FDA registration fees have to be paid to complete the registration process. Notably, the fees are subject to periodic revisions and are a significant aspect of compliance for these establishments. Thus, approval from the FDA is necessary in the medical device sector in the USA for operations to commence.
Who needs US FDA medical devices establishment registration?
- Manufacturers: Any entity manufacturing finished medical devices for commercial distribution in the U.S.
- Initial distributors: Entities responsible for first distributing the device domestically.
- Re-packagers and re-labelers: Companies that modify packaging or labeling before distribution of medical device.
- Importers and exporters: Companies importing or exporting medical devices into or out of the U.S.
- Medical device processing: Entities who perform various medical device processing stages, such a sterilization.
Establishment registration: Key aspects to keep in mind
- Electronic submission: All medical devices establishment registration and listing information has to be submitted electronically unless a waiver is granted.
- Medical device listing: Registered medical device establishments are required to list the medical devices they manufacture and the activities performed on them.
- Annual registration fee: Establishments must pay an annual registration fee. The registration fees are subject to periodic revisions.
- Premarket submission: If the medical device requires an FDA approval prior to marketing, the entity involved must provide the premarket submission number (e.g., 510(k), De Novo, PMA, PDP, HDE) along with their registration.
- Public health preparedness: Medical devices establishment registration helps FDA gauge the public health preparedness. It helps the FDA keep track of where the medical devices are made, thereby improving FDA’s ability to respond to public health emergencies.
Need help with establishment registration & device listing?
Get in touch
Medical Device Listing Mandate
Registered medical device establishments are required to list the medical devices they manufacture. Organizations must provide information on the devices they manufacture. The premarket submission number, such as US FDA 510(k), premarket approval (PMA), humanitarian device exemption (HDE), etc., has to be furnished, if the device necessitates premarket procedures. Notably, an initial importer must register their establishment but is not required to list the devices. An entity who is responsible for marketing the device entering the US from a foreign manufacturer to the final distributor for sale of the device to the ultimate consumer is considered the “initial importer”.
US FDA Medical Devices Establishment Registration: Exemptions
As per 21 CFR 807.65, certain entities are exempt from the US FDA medical device registration requirements.
- General-purpose laboratory equipment manufacturers (if the device is not labelled or marketed for medical use)
- Manufacturers of raw materials or components used in medical devices (entities who do not directly manufacture the final medical device).
- Veterinary device manufacturers (devices used exclusively for animals).
- Licensed practitioners, such as physicians, dentists, and optometrists, who modify or manufacture devices solely for use in their practice.
- Retail establishments, such as pharmacies, surgical supply outlets, that sell devices directly to consumers.
- Research, teaching, or analysis facilities that manufacture devices only for internal use and do not distribute them commercially.
- Carriers, such as shipping companies, that transport medical devices but do not manufacture or distribute them.
- Dispensers of medical devices, such as hearing aid dispensers, opticians, and hospital personnel, whose primary role is to provide a service rather than manufacture devices.
Notably, certain low-risk devices are exempt from premarket notification (510(k)) requirements. However, manufacturers of these low-risk devices are still required to register their establishments. If you are not sure whether you are exempt from US FDA medical device registration requirements, feel free to reach out to us.
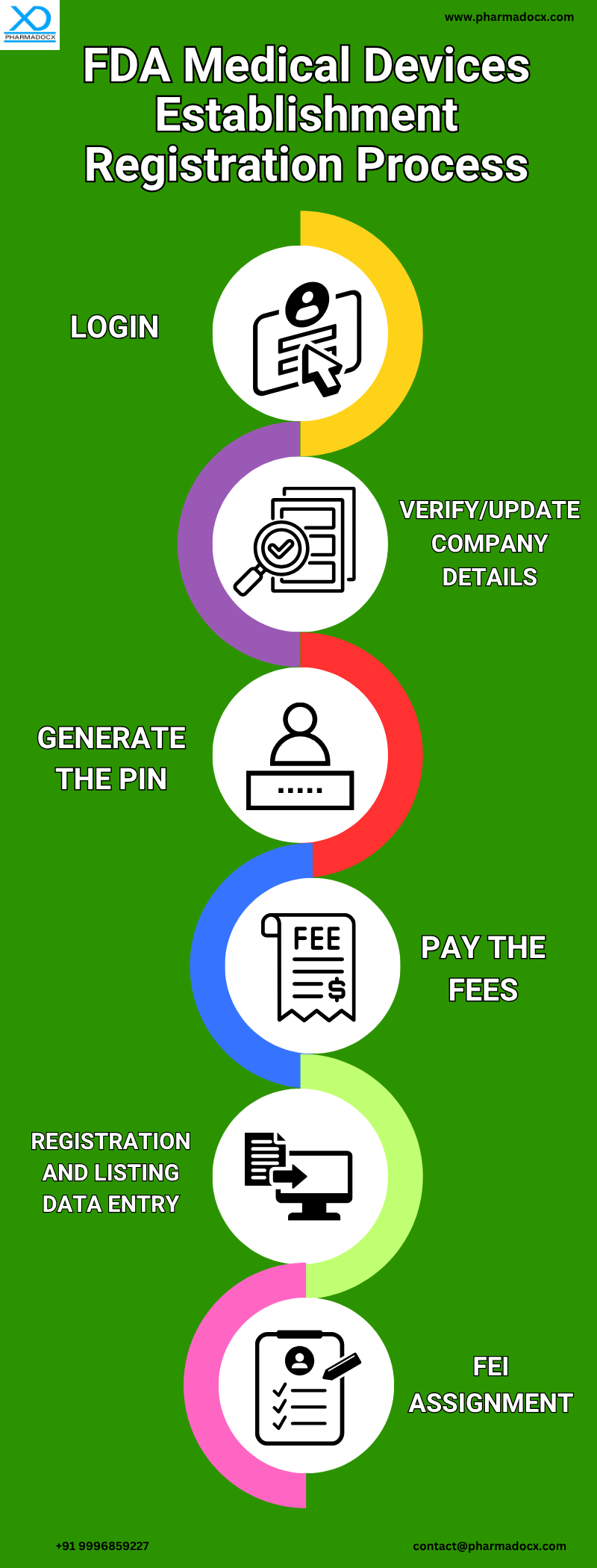
US FDA Medical Devices Establishment Registration Process: A Step-by-step Guide
- Login: Create/log in to the facility user fee account.
- PIN generation: Verify/update company details and generate the Payment Identification Number (PIN)
- Pay the fees: Electronically pay the annual registration user fee on the device facility user fee (DFUF) website.
- Registration and listing data entry: Registration and listing data is entered using the US FDA’s Unified Registration and Listing (FURLS) or the Device Registration and Listing Module (DRLM).
- FEI assignment: After the registration process is complete, the US FDA assigns an FDA Establishment Identifier (FEI) and issues the certificate via the FDA Listing Inc.
Consequences of Failing to Register with the FDA
If a medical device establishment fails to register with the FDA, it can face serious consequences. We have listed some of them.
- Misbranding of devices: Medical devices manufactured at an unregistered medical device establishment are considered misbranded under Section 502(o) of the Federal Food, Drug, and Cosmetic Act.
- Import restrictions: The FDA may detain devices from foreign establishments that fail to register. These devices maybe prevented from entering the U.S. market.
- Warning letters and enforcement actions: The FDA can issue warning letters, conduct inspections, and impose penalties on non-compliant establishments.
- Legal consequences: Failure to register the medical device establishment can lead to civil or criminal actions, including injunctions and prosecution.
- Business disruptions: If an establishment does not renew its registration annually between the stipulated period, 1st October and 31st December, it may face suspension. This will disrupt sale and distribution of the medical devices, thereby disrupt the business.
Pharmadocx Consultants: Easily Navigate US FDA Regulatory Requirements
We have extensive knowledge of the US FDA medical device regulatory guidelines. Our team will help you easily navigate the US FDA regulatory requirements, prepare the necessary documents, and submit your application. For any queries or support with US FDA medical devices establishment registration and device listing, drop an email at [email protected] or call/Whatsapp on 9996859227.
Let's Talk!
We'd love to hear from you! Whether you have questions about our pharmaceutical plant setup consultation services or want to discuss a potential project, our team is here to help. Simply fill out the form below, and we'll get back to you as soon as possible. Alternatively, you can reach out to us directly using the phone number or email address listed on this page. We look forward to connecting with you!
Phone / Whatsapp
Address
- Head Office - Opposite Dewan Mill, Old D.C. Road Sonepat - 131001 Haryana, India
- Registered Office - Netaji Subhash Place, Delhi, 110034
