US MoCRA Guidelines for Cosmetics Industry: 8 Key Provisions
With the implementation of Modernization of the Cosmetics Regulation Act (MoCRA), the FDA has unprecedented authority over the cosmetics industry. MoCRA has set stringent quality and regulatory standards for the cosmetics industry. Thus, compliance with the MoCRA guidelines is mandatory for cosmetics business to market their products in the US.
Hence, a thorough knowledge of the MoCRA requirements is necessary. Our team has extensive expertise in the US FDA regulations for the cosmetics industry and MoCRA requirements.
Pharmadocx Consultants team will help you easily navigate the MoCRA regulations from facility registration to adverse event reporting.
With our support, MoCRA compliance will be a cake walk for you.
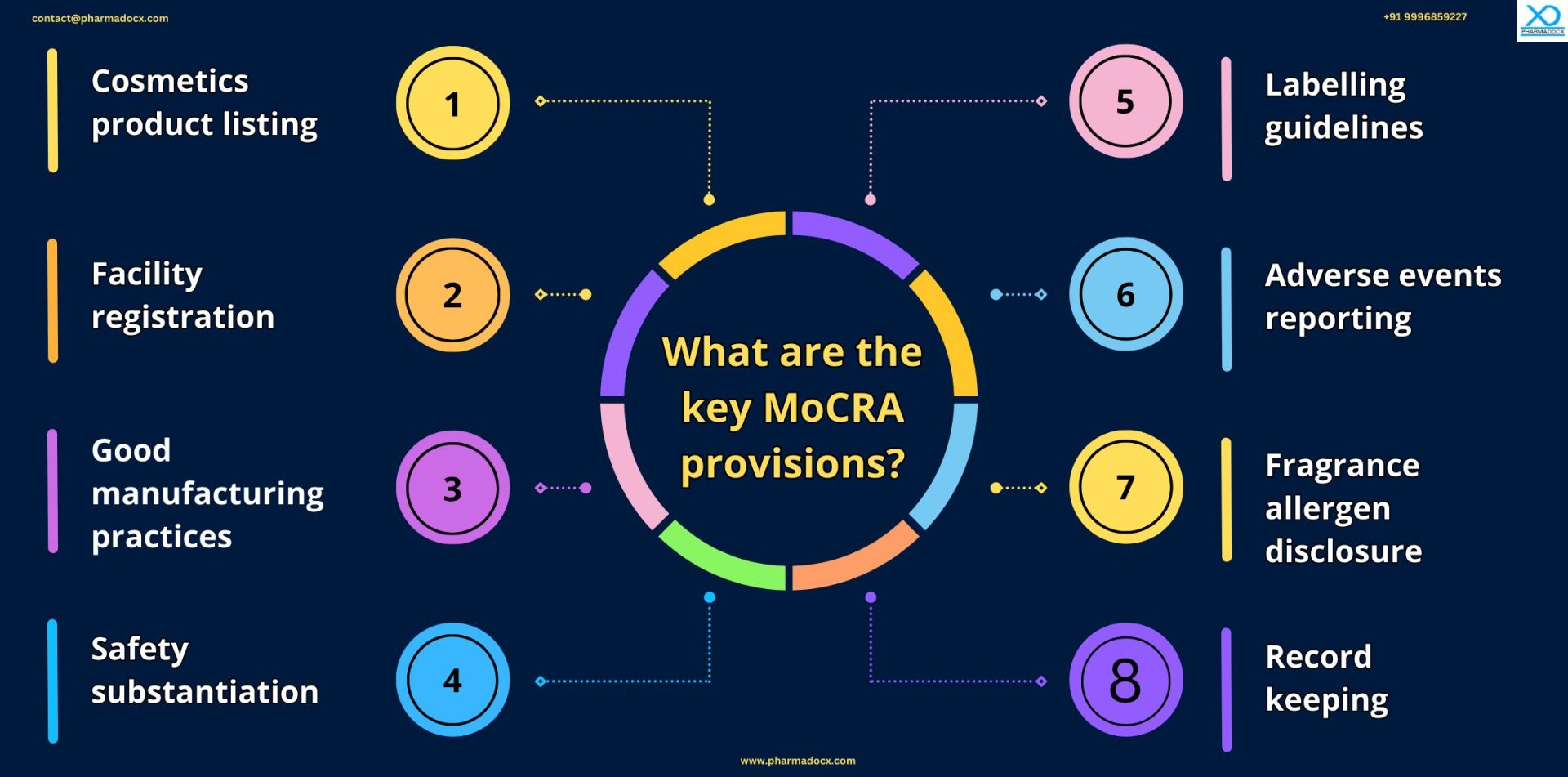
What are the MoCRA guidelines?
Implementation of MoCRA guidelines will safeguard consumer health by improving transparency and quality control measures and strengthening reporting guidelines.
Cosmetics product listing
The cosmetics product companies have to submit a comprehensive list of all marketed products to the FDA within 1 year of MoCRA’s enactment. Furthermore, for products marketed after the MoCRA enactment, a complete listing has to be submitted to the FDA within 120 days of marketing. Moreover, the responsible person will be required to annually update each marketed cosmetic product with the FDA.
Cosmetics facility registration
All existing cosmetics facilities have to be registered with the FDA by December 29, 2023. Additionally, new cosmetics facilities must register on the FDA platform within 60 days of inception. Moreover, the cosmetics facility registration has to be renewed every 2 years.
Safety substantiation
The MoCRA guidelines require the responsible person to maintain records proving their cosmetics product’s safety and efficacy. The substantiation records can include tests, studies, or analyses deemed sufficient by qualified industry experts.
Cosmetic good manufacturing practices
The good manufacturing practices (GMP) guidelines have been formulated to ensure high-quality cosmetics are consistently manufactured. Thus, the production of adulterated cosmetics can be avoided. MoCRA guidelines require cosmetics facility to abide by cosmetics GMP guidelines.
Cosmetics labelling requirements
Contact information for reporting adverse effects has to be mandatorily mentioned on the cosmetics product label. A domestic address, phone number, or e-contact information where the adverse events can be reported by consumers should be mentioned. Additionally, fragrance allergens have to be flagged separately on the label. Moreover, professional cosmetics must be clearly labelled as intended for use only by licensed professionals.
Adverse event reporting
MoCRA guidelines mandate the responsible person to promptly report any serious adverse event that may have occurred upon the use of the cosmetics product in the U.S. This should be reported to the FDA within 15 business days. Moreover, additional medical information related to the initial report must be shared for up to 1 year.
Fragrance allergen disclosure
MoCRA guidelines require that any product ingredient deemed an allergen by the FDA must be listed and fully disclosed on the product label. Each product has to be evaluated using scientific studies to prove they pose no harm to consumers.
Mandatory record-keeping requirements
MoCRA requirements mandate maintaining adverse event records associated with the cosmetic product usage for 6 years (3 for small businesses). Furthermore, MoCRA guidelines empower the FDA to inspect facilities and access necessary records. Hence, it is vital to properly maintain the records.
MoCRA guidelines for mandatory product recall authority
MoCRA guidelines have bestowed FDA authorities with the power to inspect cosmetics facilities and access their records. The authorities can evaluate products for adulteration and misbranding.
Adulterated cosmetics product
As per regulatory guidelines, a cosmetic product will be considered “adulterated” under the following conditions:
- The cosmetics product has been manufactured in conditions that do not comply with the cosmetics good manufacturing practise regulations.
- The cosmetics product container is composed of any poisonous substance that may render its contents injurious to health.
- The product itself contains any poisonous substance that may render it injurious for its intended use.
- The product itself contains any putrid or decomposed component
- The cosmetics product contains a colour additive that is considered unsafe or has not been approved by the regulatory authorities.
- The product has been prepared, packed or, stored, under unsanitary or unhygienic conditions. Thus, the product has been contaminated.
Misbranded cosmetics product
As per regulatory guidelines, a cosmetic product will be considered “misbranded” under the following conditions:
- The cosmetic product has misleading product packaging
- The required information on the product label is incomplete
- The cosmetics product labelling is false or misleading
- There is improper labelling of the colour additives used in the cosmetics product
- The cosmetics product has not been packed properly
If the FDA authorities believe a cosmetic product is adulterated or misbranded and can cause serious harm to the consumer, they can demand a recall. For which, they will contact the responsible person. The responsible person will be expected to cease production of the product. Additionally, the responsible person will have to order a voluntary recall of the cosmetics product. However, if the responsible person does not choose to voluntarily recall the product, the FDA will issue a mandatory recall. The MoCRA has empowered FDA with the ability to enforce a mandatory product recall for those products they deem as adulterated, misbranded, or pose public health risks. Then, the responsible person will have no choice but to recall those products.
To easily achieve MoCRA compliance,
contact us now
MoCRA guidelines for cosmetics facility suspensions
MoCRA has bestowed FDA with the power to suspend a cosmetics facility’s registration. A cosmetics facility registration can be suspended under the following conditions:
- A cosmetics product was either manufactured or processed by a facility that has a reasonable probability of causing serious adverse health consequences to the consumers
- Other products manufactured by the particular facility has caused similar effects
Exemption from MoCRA guidelines and regulations
The MoCRA small business exemption is beneficial for small enterprises operating in the cosmetics industry. Any business whose average gross annual cosmetic products sale for the previous 3 years is less than USD 1 Million in the US is consider a small business. These enterprises are exempt from facility registration, GMP, and product listing requirements as set forth by MoCRA. Additionally, they are only required to maintain adverse event report records for 3 years.
To be eligible for the exemption, the business should not be involved in manufacturing/processing cosmetic products that can be injected. Additionally, the products should not alter appearance for more than 24 hours without consumer removal. Furthermore, they should not be intended for internal use. Moreover, they should not come into contact with the eye’s mucus membrane.
MoCRA US agent
Non-US-based cosmetics facilities will be required to appoint an in-country representative, the US agent. The US agent will be the domestic point of contact for companies based outside US. However, the role of MoCRA US agent has not yet been clearly defined by the regulatory authorities. Nevertheless, it is expected to be similar to those of food and pharmaceutical industry US agents.
FAQs on MoCRA guidelines
What is MoCRA?
Modernization of Cosmetics Regulation Act (MoCRA) has been implemented to modernize and transform the FDA cosmetic regulations. Under this act, new facility rules, product listings, SAE provisions, product labelling, and safety validations have been introduced.
What is the deadline for registering products and facilities with the FDA as per MoCRA requirements?
All existing cosmetics products and facilities must be registered with the FDA by December 29, 2023.
How do I substantiate the safety of my cosmetics products as per the new MoCRA requirements?
The FDA has not provided explicit guidelines for substantiating the safety of cosmetics products. However, MoCRA suggests referring to international standards. Notably, the substantiation records can include tests, studies, or analyses deemed sufficient by qualified industry experts.
Who is exempt from MoCRA requirements?
Small businesses whose average gross annual cosmetic products sale for the previous 3 years is less than USD 1 Million is exempt from MoCRA requirements. They are exempt from MoCRA provisions, such as facility registration, GMP, and product listing requirements.
How often do I need to renew my cosmetics facility registration per MoCRA guidelines?
As per the MoCRA requirements, facility registrations must be renewed every 2 years.
Let's Talk!
We'd love to hear from you! Whether you have questions about our pharmaceutical plant setup consultation services or want to discuss a potential project, our team is here to help. Simply fill out the form below, and we'll get back to you as soon as possible. Alternatively, you can reach out to us directly using the phone number or email address listed on this page. We look forward to connecting with you!
Phone / Whatsapp
Address
- Head Office - Opposite Dewan Mill, Old D.C. Road Sonepat - 131001 Haryana, India
- Registered Office - Netaji Subhash Place, Delhi, 110034

