CDSCO Registration for SaMD: Software as a Medical Device
Software as a Medical Device (SaMD) has to be regulated to monitor its safety, efficacy, and quality. Whether the software can serve its intended purpose of diagnosing, treating, or managing medical conditions needs to be monitored. Additionally, the ability of the software to safeguard patient information and maintain privacy needs to be verified. Sensitivity of the data involved in SaMD warrants the need for proper regulation of the software. In India, Central Drugs Standard Control Organization (CDSCO) stringently regulates Software as a Medical Device. Therefore, securing CDSCO registration for SaMD is a mandatory requirement for marketing your product in India. We at Pharmadocx Consultants will help you secure the CDSCO registration for Software as a Medical Device in a hassle-free manner.
What is Software as a Medical Device (SaMD)?
Software as a Medical Device (SaMD) is a software or technology intended to be used for a medical purpose. The software can be used for diagnosing, treating, or managing medical conditions. Notably, SaMD operates independently, fulfilling medical purposes without requiring hardware. Moreover, the SaMD itself is treated as a medical device. Hence, it requires regulatory approval and a license.
CDSCO criteria for Software as a Medical Device in India
CDSCO has formulated certain criteria to identify whether a software qualifies as a medical device. As per Medical Device Rules, 2017, a Software as a Medical Device is a software used to treat, diagnose, mitigate, cure, or prevent disease or other conditions. It can be a software technology or mobile app. It can used as a standalone technology or can function in combination with other software. Thus, a software will be considered a medical device only if it was developed with the intention of being used for medical purposes. Notably, CDSCO registration for Software as a Medical Device is a mandatory requirement for launching the software in the Indian market.
CDSCO Classification for Software as a Medical Device (SaMD)
CDSCO has classified medical devices into four classes based on the associated risk level. This classification system has been developed to simplify the CDSCO medical device license application process. The CDSCO medical device class will determine the CDSCO license required and the application process. To register a SaMD in India, CDSCO has developed a similar risk-based classification system.
CDSCO SaMD Class A
The Class A software does not directly interfere with patient data. It is a software that retrospectively analyses pre-recorded clinical data of patients. Examples:
- Continuous glucose monitor retrospective data analysis software
- Ataxiagraph with interpretive software
CDSCO SaMD Class B
The Class B software generates real time patient information based on patient’s parameters. Examples:
- Software for visual evoked response stimulator
- Software for auditory evoked response stimulator
- Software for pulmonary exercise stress monitoring system
- Software for ECG recorder with real-time analysis
- Software for film-recorded digital radiography
- Analyzing software for hemodynamics or cardiac function
- Supporting software for root canal treatment
- X-Ray angiographic imaging-based coronary vascular simulation software device
- Automated radiological image processing software
- Image acquisition and/or optimization guided by artificial intelligence
- Chairside dental CAD/CAM unit
CDSCO SaMD Class C
The Class C software can be used to diagnose diseases and analyse patient’s physiological and physical activity. Examples:
- Diabetic retinopathy detection device
- Computer-assisted diagnostic software for lesions suspicious for cancer
- Burn resuscitation decision support software
- Software, similarity score algorithm, tissue of origin for malignant tumor types
- Insulin pump therapy adjustment calculator for healthcare professionals
- Coronary vascular physiologic simulation software
- Angiographic coronary vascular physiologic simulation software
- Software for visualization of vascular anatomy and intravascular devices
- Orthodontic software
CDSCO SaMD Class D
CDSCO has not yet classified any software as high risk.
CDSCO Registration for SaMD in India
Currently, medical devices are not just physical devices. A software itself can be used as a medical device. These SaMDs are used not only to diagnose diseases but also to decide the course of treatment. Thus, it is important to verify that the software performs as intended to minimize malfunctions that could harm patients. Furthermore, as doctors use SaMDs to diagnose diseases, confidentiality of patient data and information is important. Additionally, accuracy of the data generated by the software is essential for proper diagnosis. Thus, with technological advancement, software plays a vital role in healthcare industry. Hence, owing to its increased use and impact in the healthcare industry, SaMD has to be strictly regulated.
Therefore, regulatory guidelines have been formulated to set benchmarks for ensuring the software quality, safety, and efficacy are maintained. Mandatorily securing regulatory approval of SaMD is essential for improvement of patient outcome as well as advancement of healthcare industry.
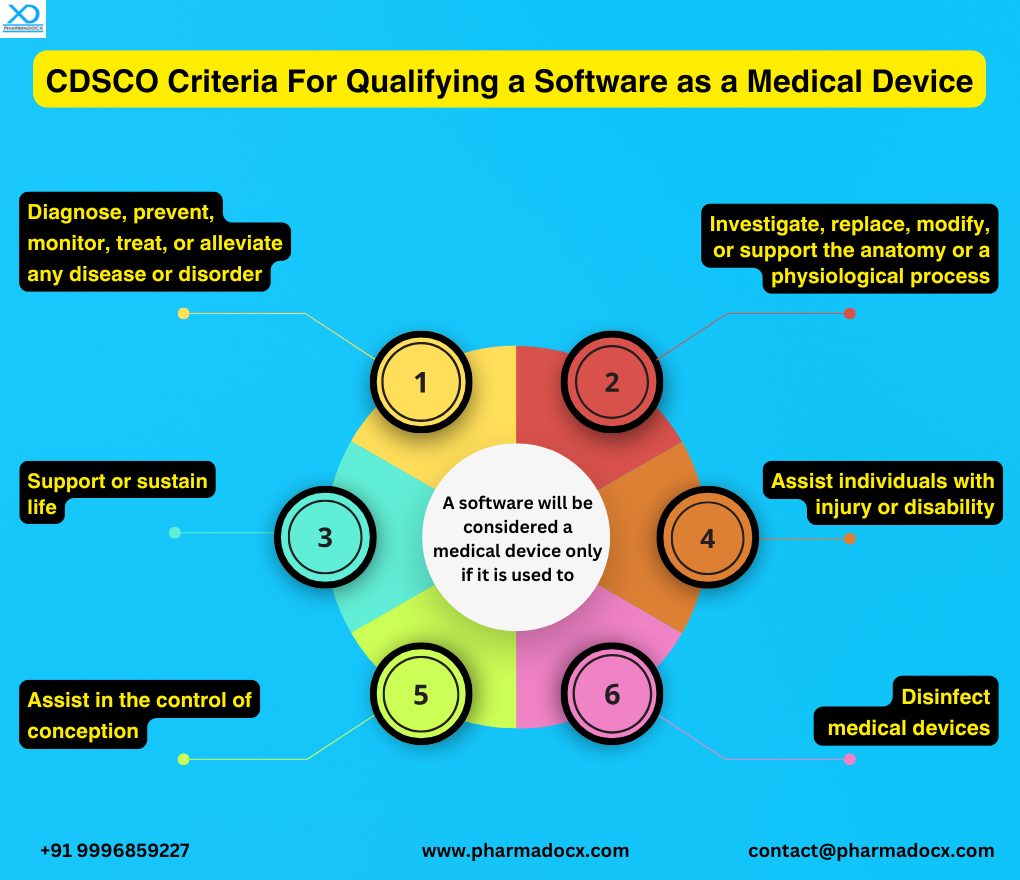
Hence, securing the CDSCO registration for SaMD is necessary to market Software as a Medical Device in India. Furthermore, as per the latest notification, instead of the mandatory registration, all medical devices, including SaMD, will now have to adhere to the licensing system. The CDSCO medical device manufacturing license will have to secured to manufacture SaMD in India. On the other hand, CDSCO medical device import license will have to obtained to import SaMD into India.
Documents required for securing CDSCO registration for SaMD in India
We have provided an overview of the documents required for securing CDSCO registration for SaMD in India.
- Sale deed/rent deed of the premises proving the legal ownership of the medical device manufacturing facility.
- Organization identity proof, such as memorandum of association, list of directors or partners, UDYAM Aadhar, or PAN card.
- Facility layout with dimension. A detailed drawing of the medical device manufacturing facility with the dimensions of each room and the location of all equipment.
- ISO 13485 certificate
- Plant master file containing information about the layout of the facility.
- Device master file contains detailed information about the software, including its design, manufacturing process, testing procedures, and validation
- Documents demonstrating compliance with the environmental requirements
- Certificate of analysis demonstrating the software meets the required quality standards
- Details and qualification of technical staff who will manufacture and test the medical devices
- Test license for the SaMD, if required
This list provides an overview of the documents required for securing CDSCO registration for SaMD in India. Feel free to get in touch for a detailed list of documents required for CDSCO SaMD license application. Additionally, we will help prepare and compile the documents per CDSCO guidelines.
CDSCO Registration for SaMD in India: License and Requirements
CDSCO license for manufacturing SaMD in India
Different classes of SaMD require different CDSCO licenses.
- To manufacture Class A and Class B Software as Medical Device (SaMD) in India, CDSCO MD 5 license must be secured from the state licensing authority.
- To manufacture Class C and Class D Software as Medical Device (SaMD) in India, CDSCO MD 9 license must be secured from the central licensing authority.
CDSCO license for importing SaMD into India
To import Software as Medical Device (SaMD) into India, CDSCO MD 15 has to be secured from the Central Drugs Standard Control Organization.
Validity of CDSCO registration for SaMD in India
The CDSCO registration for SaMD remains valid indefinitely. However, a license retention fee has to be paid every 5 years to maintain the registration validity.
How to Apply for CDSCO Registration for SaMD in India?
We have provided an overview of the process of CDSCO registration for Software as a Medical Device in India.
- Check whether the software qualifies as a medical device
- Check the CDSCO class for the SaMD, as this will determine the application form and requirements.
- Login or create an account on CDSCO’s SUGAM Portal.
- Fill in the respective application forms
- Compile and upload the necessary supporting documents. Additionally, pay the required fees.
- The application will be reviewed and the documents will be verified by the regulatory authorities. Furthermore, the regulatory authorities may conduct an audit.
- If the regulatory officials are satisfied and no non-compliance is observed, then the application will be approved. Then, the CDSCO license for manufacturing or importing the SaMD will be granted.
We have provided a basic outline of the CDSCO SaMD license application process. For CDSCO SaMD registration and license application support, feel free to contact us using the form alongside. We have extensive expertise and knowledge of the CDSCO medical device license application process. Our team of experts has more than 27 years of industry experience. We will be more than happy to help you easily obtain CDSCO registration for Software as a Medical Device in India. Our holistic support covers everything from document preparation to application submission. Moreover, our support does not end with license application. We will provide continued support till you successfully secure the CDSCO license.
Need help with CDSCO registration for SaMD?
Fill out the form below
Why choose Pharmadocx Consultants?
Drugs Licences
Years Experience
Plants Set-up
Our Clients

Navigating the CDSCO regulatory guidelines can be a tricky task. We will help you secure the CDSCO registration for SaMD in a hassle-free manner. Drop an email at [email protected] or call/Whatsapp on 9996859227 and we will take care of all the regulatory headaches.
Let's Talk!
We'd love to hear from you! Whether you have questions about our pharmaceutical plant setup consultation services or want to discuss a potential project, our team is here to help. Simply fill out the form below, and we'll get back to you as soon as possible. Alternatively, you can reach out to us directly using the phone number or email address listed on this page. We look forward to connecting with you!
Phone / Whatsapp
Address
- Head Office - Opposite Dewan Mill, Old D.C. Road Sonepat - 131001 Haryana, India
- Registered Office - Netaji Subhash Place, Delhi, 110034

