New-age technology and software are revolutionizing healthcare. Currently, software is being used to monitor, diagnose, prevent, manage, and even treat diseases. Software used in healthcare industry are available in different types and forms. The two main categories of software used in healthcare industry are Software as a medical device (SaMD) and Software in a medical device (SiMD). Although these terms are used interchangeably, they have distinct differences. Herein, we will focus on the differences between SaMD and SiMD and the different regulatory requirements for the two categories.
Healthcare regulators worldwide have developed policies to regulate software being used in the healthcare industry. India’s medical device regulatory body, CDSCO, also has regulations for the software. Software is regulated to protect the public health in India. Additionally, regulatory approval for medical device software is required to ensure its quality and efficacy and protect patient data.
Understanding SaMD and SiMD
Before understanding differences between SaMD and SiMD, let us focus on the definition of these two main categories of software.
Software as a Medical Device (SaMD)
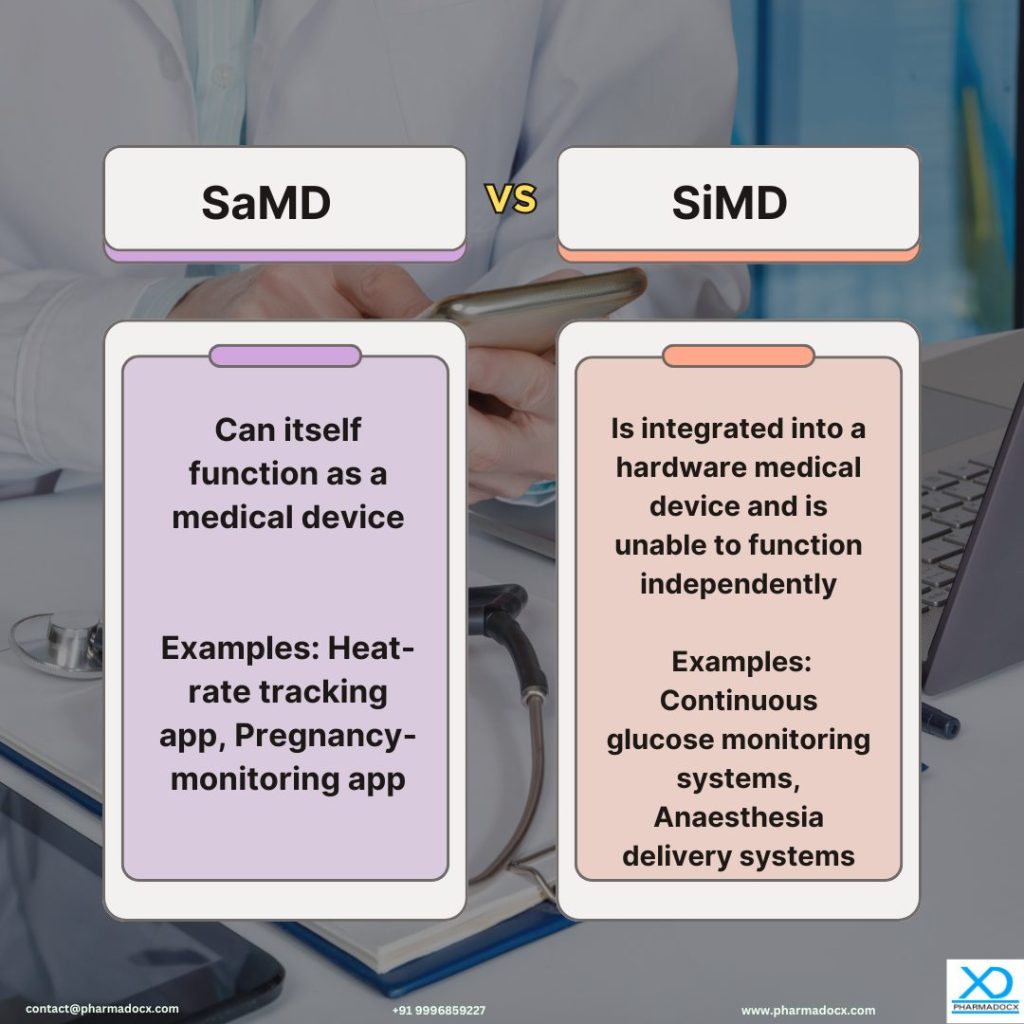
SaMD is the software intended to be used for medical purposes, such as diagnosis, monitoring, and treatment. SaMD runs on various platforms without being part of a hardware medical device. The software can function independently to provide medical information or perform medical functions.
Examples:
Heat-rate tracking app: A health device used to monitor the heart-beat rate; Pregnancy-related app: A software intended to support the user’s conception by calculating her fertility status; Remote patient monitoring systems: Platforms used by healthcare providers to monitor patients’ vital signs; Medical imaging analysis software: Software used to analyse medical images, such as X-rays, CT scans, or ultrasounds for detecting abnormalities; Cognitive behaviour software: Technology used to offer cognitive behavioural therapy.
Software in a Medical Device (SiMD)
SiMD is the integral component or accessory of a hardware medical device and is vital for proper functioning of the device. However, it cannot function independently and depends on the associated medical hardware. It contributes to the overall medical device system operation. Notably, any malfunction or failure of the SiMD could compromise the safety and performance of the medical device.
Examples:
Automated external defibrillators: These devices contain embedded software that analyse heart rhythms and deliver electric shocks to restore the heartbeat to normal; Continuous glucose monitoring systems: Devices with embedded software that collect real-time data to measure and monitor glucose levels in diabetic patients; Anesthesia delivery systems: These devices administer anesthesia during surgery based on its embedded software ensuring precise control and patient safety. Pacemakers and implantable cardioverter defibrillators: Using data from its embedded software, these devices monitor and regulate heart rhythms. Infusion pumps with dose-calculator: Based on appropriate dosage calculated by the embedded software as per patient parameters, the device delivers controlled doses of medication.
Differences between SaMD and SiMD
We have provided an overview of SaMD vs SiMD. The differences between the two form the basis of differences in patient safety and regulatory compliance.
Development and validation of the software
Development, validation, and verification of SaMD are based on software-specific considerations. The reliability and accuracy of the medical information and functionality of the software need to be kept in mind. The SaMD platform’s memory, CPU, and other elements considerably impact the performance of the software. Hence, these factors should also be considered while developing the SaMD.
In case of SiMD, coordinated development between hardware and components is required. Thus, rigorous validation and testing are required to ensure proper integration and functionality of the software in hardware medical device.
Patient safety
To ensure safety of patients using SaMD, regulatory compliance is a must. For patients to trust the safety and reliability of SaMD, stringent regulations and guidelines are required.
In case of SiMD, effective functioning and proper integration of software are pivotal for patient safety. The proper functioning of the device depends on the software. Hence, stringent guidelines monitoring the proper integration of the software into hardware medical device will improve patient outcome and safety.
Risk management
Developers of SaMD need to analyse the risks associated with it. The safety, quality, and effectiveness of SaMD in delivering its intended medical purpose need to be thoroughly analysed.
Given the impact of SiMD, comparatively in-depth risk analysis of these devices is necessary. The developers of SiMD need to ensure the safety and performance of devices integrated with software are not compromised.
Regulatory compliance for SaMD and SiMD
In India, both SaMD and SiMD are subject to CDSCO medical device regulations. Owing to the important role they play in the medical devices industry, CDSCO has set stringent regulatory guidelines for them. These regulations monitor the safety, efficacy, and quality of SiMD and SaMD. It is important to note regulations for these devices vary from country to country. SaMD is usually regulated as a standalone medical device. Whereas SiMD maybe regulated along with its associated hardware device.
Both SiMD and SaMD are classified as per CDSCO risk-based classification system. IEC 62304, IEC 62366, and ISO 14971 are applicable to both SaMD and SiMD. Few differences are there in the safety and regulatory standards that are applicable to SaMD and SiMD. SiMD has to meet regulations concerning the safety and performance of the integrated software as well as overall medical device. Additional requirements related to the device hardware and proper software integration are applicable to SiMD.
SaMD vs SiMD regulatory documentation requirements
Following documents are usually required to demonstrate regulatory compliance:
- SaMD documentation: Documents demonstrating clinical validation evidence, software development lifecycle, risk management processes, and post-market surveillance protocol compliance are required.
- SiMD documentation: Documents providing evidence of compatibility between the software and associated hardware device. Furthermore, proof of software-hardware interface validation and risk assessments of software-hardware interactions is required.
Importance of regulation of software being used in the healthcare industry
In simple words, SaMD is a software which is itself a medical device. On the other hand, SiMD is a software which is part of another medical device. Understanding the differences between SaMD and SiMD will help understand the applicable regulatory requirements. It is important to verify that the software performs as intended to minimize malfunctions that could harm patients. As doctors use SaMD and SiMD to diagnose diseases, confidentiality of patient data is important. Accuracy of the data generated is essential for proper diagnosis. Proper regulatory compliance is necessary to ensure patient safety and protect public health. Additionally, regulatory compliance will provide access to the medical device market without any regulatory hiccups.
Pharmadocx Consultants: Your trusted regulatory ally
CDSCO registration is necessary to market medical devices, including SaMD and SiMD, in India. Understanding the CDSCO regulatory guidelines is not easy. Pharmadocx Consultants can provide the necessary regulatory support to secure CDSCO registration and medical device license. We have served over 600 clients and have more than 27 years of experience. Pharmadocx Consultants can help you navigate through the complex registration process for medical devices in India. Drop an email at [email protected] or call/Whatsapp on 9996859227 and we will get the CDSCO medical device registration process started.





0 Comments