CDSCO Manufacturing License for IVD Products Class A & B - MD5
Skip the confusion, delays, and rejections. Our team has guided over 300 medical device companies through CDSCO’s regulatory process for IVD Manufacturing, and we’re excited to do the same for yours.
Expert consultation with industry leaders
Fast & hassle-free approvals
Manufacturing Registration on Form MD5
Fast and accurate query replies
600+ Granted Approvals 27+ Years Experience
300+ Happy Clients












In-vitro diagnostic devices (IVDs) have a crucial role in patient diagnosis. They are used to understand the current state of health to determine the course of treatment required. Furthermore, IVD devices are used for testing patients in the primary health care facilities where labs are not available. Hence, IVDs have an indispensable role in patient diagnosis and care. Thus, they have to be strictly regulated and monitored. CDSCO has formulated strict guidelines for IVDs in India. This apex regulatory body is aimed at enforcing these regulations to monitor the quality, efficacy, and safety of IVDs. Hence, compliance with Indian IVD regulations is mandatory for all devices entering the Indian market. CDSCO has categorised IVDs into various classes for ease of licensing. Different license types are applicable for different CDSCO IVD classes. We at Pharmadocx Consultants will help you easily secure the CDSCO license for manufacturing Class A and B IVDs.
What are IVDs?
IVDs or in vitro diagnostic devices are basically instruments, reagents, kits, and systems used for diagnosing diseases and health conditions. The term “in-vitro” means in glass. The IVD tests are usually performed in test tubes or other similar equipment outside the body. IVDs are used to collect and examine specimens obtained from the human body. Basically, they are used to understand the current state of health of the patient to determine the course of treatment or next course of action required. IVD tests can be performed using various instruments from small handheld devices to complex laboratory equipment. Moreover, these tests can be performed at the patient’s homes, laboratories, or health care facilities. Owing to the impact of IVDs on the healthcare system, they have to be strictly monitored and regulated.
CDSCO Regulations for Manufacturing IVDs in India
Central Drugs Standard Control Organisation (CDSCO) regulates the manufacture, import, and sale of IVDs in India. CDSCO IVD regulations provide guidelines for IVD manufacturers to ensure the devices are safe and effective.
CDSCO definition for IVDs: IVDs are tools/substances intended to be used on specimens derived from humans for the diagnosis of any diseases or disorder in human beings.
Medical Device Rules, 2017, has laid down the risk-based CDSCO IVD classification system for simplifying the licensing process. Different regulatory guidelines and license types are applicable for different CDSCO IVD classes. IVD manufacturers must apply for the CDSCO IVD manufacturing license depending on the applicable CDSCO IVD class. Moreover, they must comply with all the CDSCO IVD regulations to avoid regulatory sanctions. CDSCO has the power to take corrective measures in case of any non-compliance.
Need help securing the CDSCO license for IVDs?
Get in touch using the form below
CDSCO IVD Classification System
CDSCO has categorised IVDs into 4 classes to simplify the CDSCO IVD manufacturing license application process. The CDSCO IVD classification system is based on the intended use and risk associated with the device.
- Class A IVD devices: These are low risk devices.
- Class B IVD devices: These are moderate risk devices.
- Class C IVD devices: These are high risk devices.
- Class D IVD devices: These devices have the highest risk.
What are Class A and B IVDs?
CDSCO class A IVD devices
CDSCO class A IVD devices are low risk devices having the following intended use:
- A substance/object with specific characteristic that make it suitable for an IVD procedure associated with a certain examination.
- A device that is specifically to be used for an IVD procedure.
Examples: Reagents and solutions to be used for laboratory testing.
CDSCO class B IVD devices
CDSCO class B IVD devices are moderate risk devices having the following intended use:
- IVD devices intended for self-testing. The devices should be used to obtain preliminary test results that need confirmation using appropriate laboratory tests. These IVD test results should not be used for the final diagnosis of a medically critical status.
Examples: Blood glucose test strips, pregnancy test kits, and urine analysis test strips.
CDSCO License for Manufacturing Class A and B IVDs in India
- Manufacturing license: The CDSCO license for manufacturing Class A and B IVDs is MD 5. The applicant will have to use MD 3 to apply for a license to manufacture, sell, and distribute Class A and B IVD devices in India. The license to manufacture Class A and B IVD devices for sale and distribution in India will be granted under MD 5.
- Loan license: A loan license permits a company without a manufacturing facility to manufacture IVDs. With the loan license, the company can use the manufacturing facility of another company that is manufacturing the same product. The applicant will have to use MD 4 to apply for a loan license for Class A and B IVD devices. The loan license to manufacture Class A and B IVDs without a manufacturing facility will be granted under MD 6.
Necessary Supporting Documents for CDSCO License Application
Certain supporting documents are required for securing the CDSCO license for manufacturing class A and B IVDs. These documents are required to demonstrate compliance with CDSCO regulations.
- Cover letter
- Organization identity proof, such as UDYAM Aadhar, PAN card, etc.
- Sale Deed/Rent Deed proving legal ownership of the manufacturing facility.
- Facility building layout presenting the dimensions of each room and the location of all the equipment in the manufacturing facility.
- Plant master file
- Device master file
- Audit reports
- ISO 13485Certificate validating the manufacturing facility meets international criteria for quality management systems.
- Certificate of analysis of 3 consecutive batches to certify the first 3 batches of the manufactured devices meet the required quality benchmarks.
- Document supporting compliance with the environmental regulatory requirements.
- Test license (if applicable)
- In case of loan license application, consent letter from the principal manufacturing unit, principal manufacturer’s license and product permit, and wholesale license of the applicant.
- Documents proving the manufacturing team comprises competent, qualified, and experienced staff who will manufacture and test the devices.
The above list provides an outline of the supporting documents required. For a detailed list of documents or for document preparation support, feel free to reach out to us.
How to Secure the CDSCO License for Manufacturing Class A and B IVDs? A Step-by-Step Guide
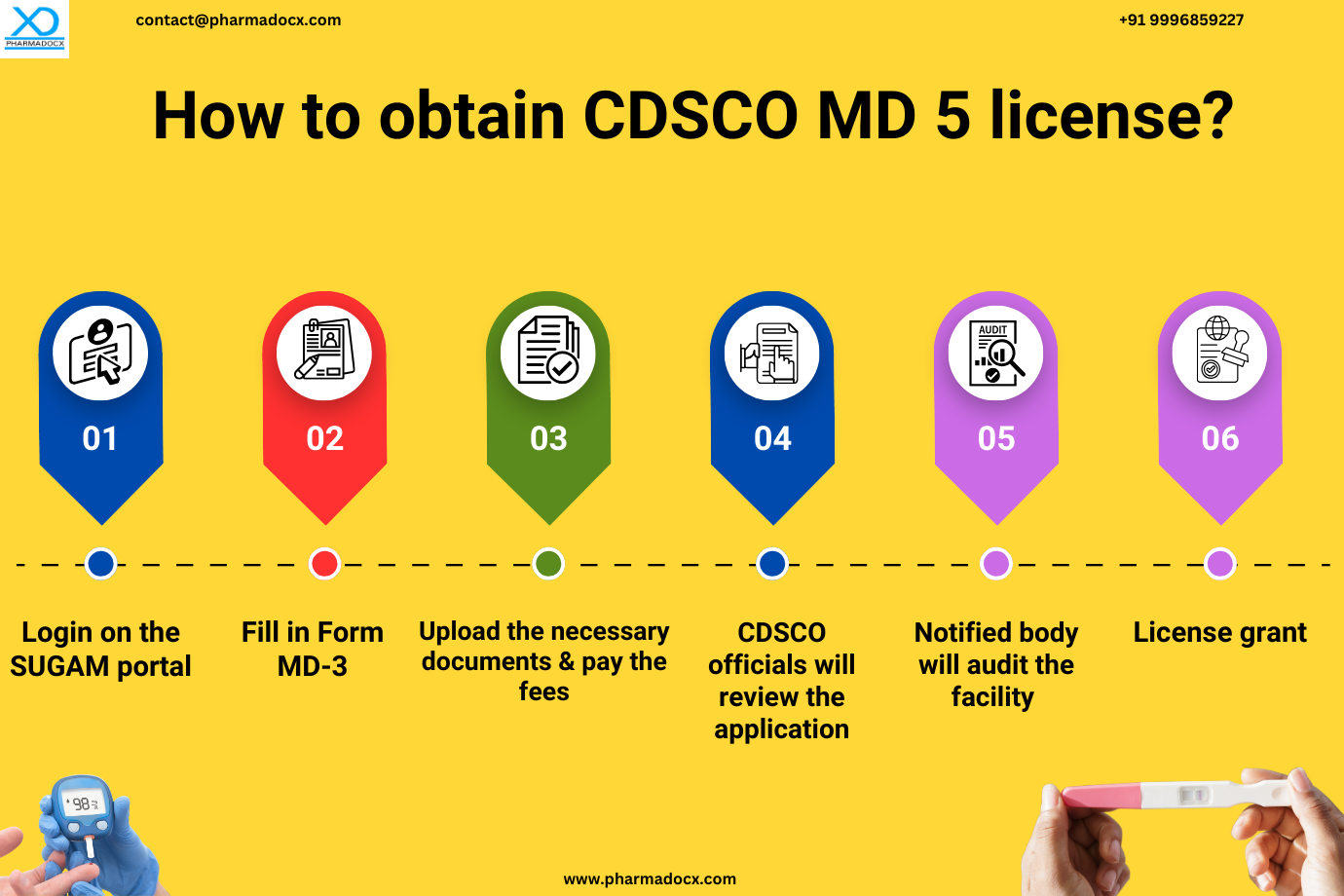
How can Pharmadocx Consultants Help?
Invitro diagnostic devices have a critical role to play in the healthcare industry. They are crucial for diagnosis diseases, providing prognosis, and monitoring progress. Hence, Indian regulations for IVDs are in place to ensure patient safety, protect public health, and improve patient outcome. CDSCO IVD regulations monitor the safety, quality, and efficacy of these devices. Moreover, these regulations ensure manufacturers focus on risk identification and reduction for IVDs. Furthermore, the IVDs should perform as intended for effective healthcare delivery. Notably, the regulations are in place to ensure safe and effective devices reach patients. Hence, having a valid CDSCO license demonstrates commitment to patient safety and delivering high-quality devices. Additionally, it ensures that healthcare professionals are equipped with the best tools for patient care. Therefore, mandatory CDSCO licensing for IVDs is in the best interest of patients and healthcare providers as well as improves brand credibility.
We provide comprehensive services tailored for IVD manufacturers
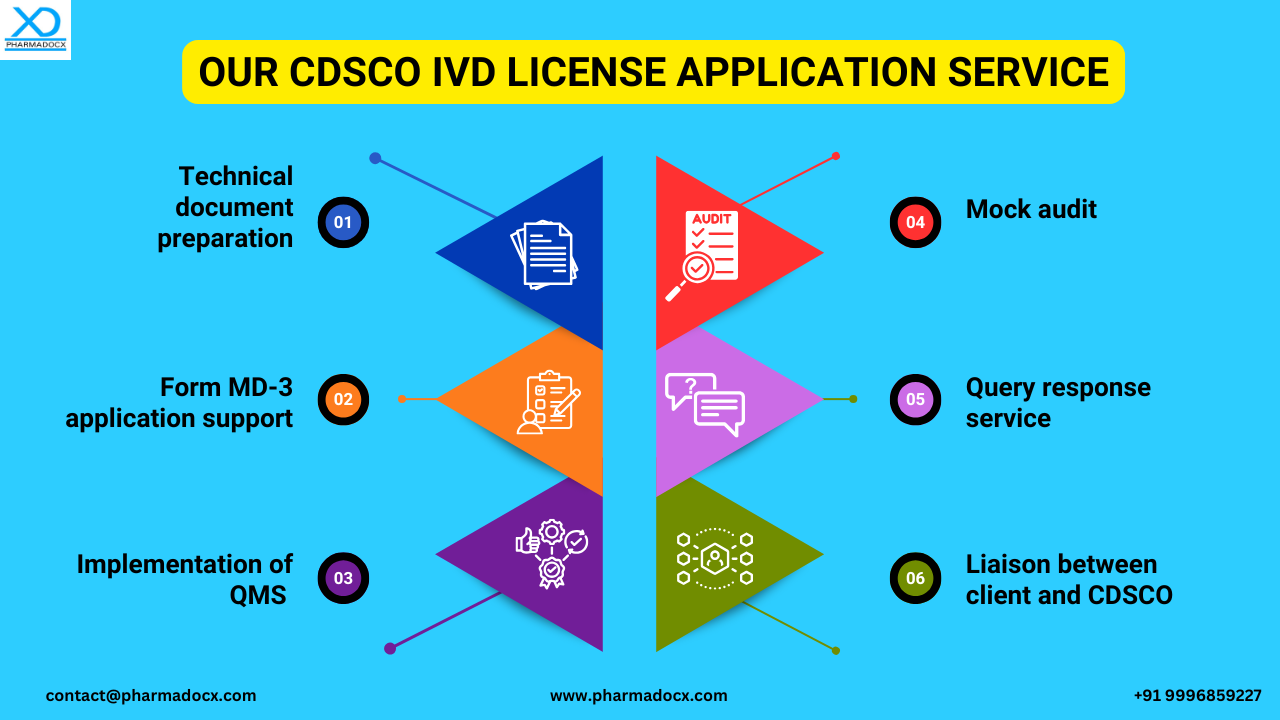
Avail our CDSCO IVD manufacturing license application service to have a smooth hassle-free regulatory journey. We will leverage our expertise to help you easily secure the CDSCO license for manufacturing Class A and B IVDs. For swift license grant and approval, call/Whatsapp us at 9996859227 or write to us at [email protected].
Let's Talk!
We'd love to hear from you! Whether you have questions about our pharmaceutical plant setup consultation services or want to discuss a potential project, our team is here to help. Simply fill out the form below, and we'll get back to you as soon as possible. Alternatively, you can reach out to us directly using the phone number or email address listed on this page. We look forward to connecting with you!
Phone / Whatsapp
Address
- Head Office - Opposite Dewan Mill, Old D.C. Road Sonepat - 131001 Haryana, India
- Registered Office - Netaji Subhash Place, Delhi, 110034

