Surgical sutures are essential for performing most surgeries. These medical devices are designed to facilitate the healing process. Most surgical or traumatic wound closure would require sutures. The increase in number of surgeries performed in India is boosting the Indian surgical suture market growth. Thus, medical device companies are focusing on manufacturing this essential surgical equipment to cater to the Indian market demand.
Surgical suture: A vital medical device for surgery
Surgical sutures are used to hold the body tissues together after a surgery or an injury. It should keep the wound closed for a sufficient period so that the injured tissue is healed. The aim of this surgical equipment is that the wound should be closed even after sutures are removed or absorbed. Sutures are available in a wide variety. A needle along with a thread is required to apply a suture. This is the most common yet vital medical device for a successful surgery.
Sutures are categorised based on absorbability (absorbable vs non-absorbable), material composition (synthetic vs natural), and structure (monofilament vs multifilament). The various categories have distinct properties that make them ideal for specific clinical situations.
Ideally, the suture should be as small as possible to generate uniform tensile strength and secure the wound for the required healing time. The suture should be easy to handle, predictable, and produce minimal reaction. The choice of suture depends on the tissue characteristics, level of tension across the wound, and infection probability.
Sutures vs Stitches
Both these terms are confused easily and often used interchangeably. However, there is a difference between the two terms. The medical device used by surgeons to repair wounds is suture. On the other hand, the technique used by surgeons to repair wounds with the help of sutures is stitching.
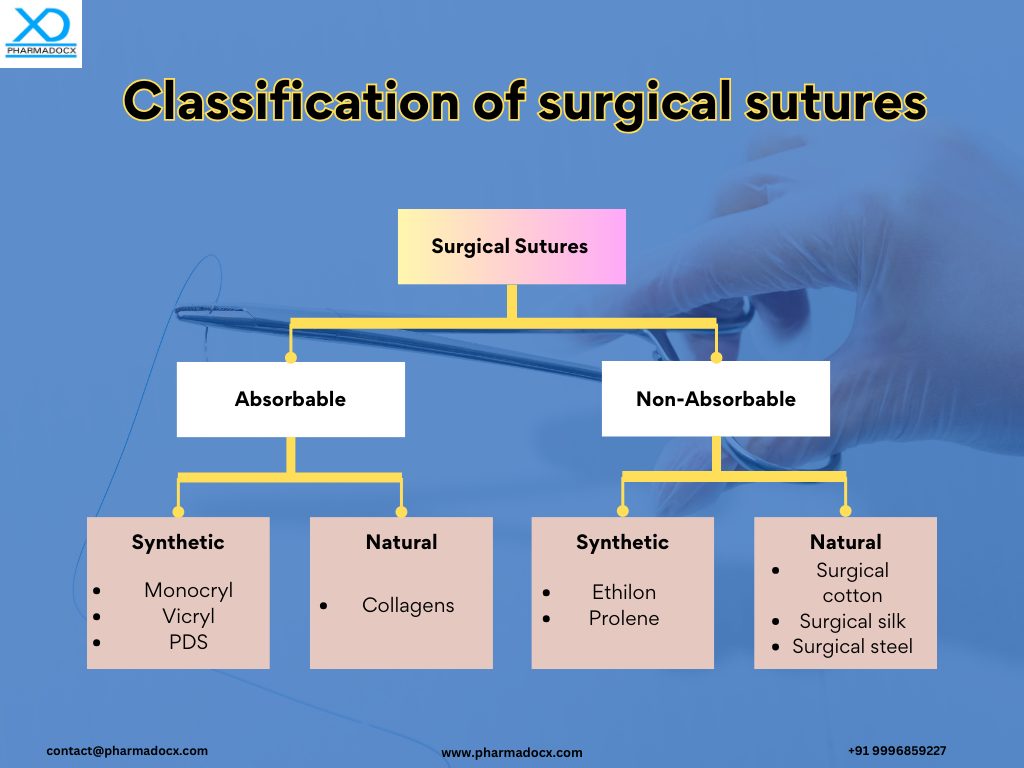
Classification of surgical sutures
Sutures can be classified based on different parameters. First, the absorbability of the suture. Second, the material composition. Third, the structure.
Absorbability
- Absorbable sutures: These sutures are broken down in the body by enzymatic digestion or hydrolysis. They are usually used for deep tissues and tissues that will heal rapidly. Absorbable sutures may be used for suturing in the urinary or biliary tracts or tying small vessels near the skin. They do not require manual removal. Factors, such as material, suture location, and patient factors, influence absorption time.
- Non-absorbable sutures: These sutures require manual extraction, as they are not naturally broken down by the body. They provide long-term tissue support. Non-absorbable sutures are used for tissues that heal slowly. They are mainly used for tendons, abdominal wall closure, and vascular anastomoses.
Material composition or origin
- Natural: Sutures constructed using materials of natural origin. Silk or catgut is used to manufacture these sutures. They are used for securing surgical drains. However, they may induce tissue reactions.
- Synthetic: Sutures constructed using man-made materials, such as polydioxanone (PDS) or nylon. Synthetic sutures have more predictable tensile strength and elasticity loss and absorption capacity than natural sutures.
Structure
- Monofilament: These sutures are constructed using a single stranded filament suture, such as nylon, PDS, or prolene. They have a low risk of infection. However, these sutures have poor knot security and ease of handling.
- Multifilament: These sutures are constructed using multiple filaments that are twisted together, such as braided silk or vicryl. Although they are associated with infection risk, they have good knot security and ease of handling.
Factors to consider while selecting sutures
- Type of tissue on which surgery will be performed
- Time period of support required for wound closure
- Allergies and sensitivities
- Risk of infection
Surgical procedures may have specific suture requirements
- Cardiovascular surgery: Non-absorbable and high-tensile strength surgical sutures, such as polypropylene or polyester, are required.
- Orthopaedic surgery: As dense and load-bearing tissues are involved in these surgeries, robust and non-absorbable materials, such as polyethylene, are required sutures.
- General surgery: Versatile synthetic sutures, such as polydioxanone or polyglactin, are required for these procedures.
- Dermatological procedures: Fine and cosmetic-friendly sutures, such as monofilament nylon or polypropylene, are required.
Indian surgical suture market landscape
As of 2024, the Indian market size for surgical sutures is estimated at USD 184.73 million. The market is expected to grow at a compound annual growth rate (CAGR) of 4.55% from 2024 to 2029. By 2029, the market size is expected to be valued at USD 230.01 million. An increase in the number of surgeries performed in India has positively influenced the demand for sutures. This has positively impacted the Indian surgical suture market growth.
Multiple factors are fuelling the boom in the Indian surgical suture market. A recent article published in World Journal of Surgery, January 2021, has highlighted the markedly high number of surgeries performed in India. Approximately 3,646 surgeries were performed annually in India as opposed to approximately 5,000 surgeries per 100,000 individuals performed globally.
India is witnessing a high incidence of chronic diseases requiring surgeries and road accident-related trauma injury. With the high number of surgeries performed in India, there is a high demand for sutures. Major surgeries, such as orthopedic, alimentary, and ophthalmic, performed in India all require surgical suturing. Additionally, bariatric surgery, which also requires suturing, is now being commonly performed in India to tackle obesity.
Hence, all these factors are fuelling the demand for surgical sutures in India. However, certain factors, such as needle-related infections and increasing preference for minimally invasive surgeries, may impede the growth of this medical device market segment.
It is important to note that the Indian surgical suture market is fairly competitive. Several major players control this medical device market segment, which has high potential.
The Indian surgical sutures market can be categorised into the following segments:
- Product type: absorbable sutures (natural sutures and synthetic sutures) and non-absorbable sutures (nylon, prolene, and other non-absorbable sutures)
- Application: orthopedic surgery, ophthalmic surgery, cardiovascular surgery, neurologic surgery, and others
- End-users: hospitals, clinics, and ambulatory surgical centres
Studies have reported the number of orthopedic and ophthalmologic surgeries is expected to grow over the next five years. These two divisions of surgery are mainly expected to drive the demand for sutures over the next 5 years.
Manufacturing surgical sutures in India
As stated above, the Indian surgical sutures market has high potential. Medical device companies in India want to focus their business on this crucial surgical equipment.
Sutures are designed to maximize wound healing and improve scar aesthetics. It is important to be aware of the various categories and types of surgical sutures that are used in the healthcare industry. Additionally, surgeons will choose sutures depending on the requirements of the surgery they will perform. Thus, a knowledge of the sutures in demand is of importance to start a surgical suture manufacturing business in India.
With the high demand for sutures in India, medical device manufacturing companies want to tap into the potential of this sector. Given the important role sutures play in wound healing, they have stringent quality control requirements. For manufacturing surgical sutures in India, you have to comply with the CDSCO regulatory guidelines.
Pharmadocx Consultants: Your trusted regulatory partner
Surgical suture manufacturing business has immense potential. Planning to launch your surgical sutures business in India? Not sure of the applicable CDSCO regulatory guidelines. Simply call/Whatsapp on 9996859227 or email at [email protected] and we will help you with all your CDSCO regulatory requirements. Our team at Pharmadocx Consultants has served over 600 clients and has more than 27 years of experience. We will leverage our expertise and industry knowledge to help you establish your medical device business in India.





