Securing the CDSCO license for gynaecological medical devices is mandatory to launch your devices in the Indian medical device market. This requirement is in place to protect the public health and maintain the standard of the Indian healthcare system. To manufacture gynaecological medical devices in India, you have to secure the CDSCO medical device manufacturing license. On the other hand, to import gynecological medical devices into India, you have to secure the CDSCO medical device import license. To secure these licenses, you have to abide by the CDSCO regulatory guidelines. In this blog, we will guide you through the process of securing the CDSCO gynaecological medical device license. Additionally, we have provided some tips to help you overcome the common CDSCO medical device license application challenges.
Complete guide to obtaining CDSCO gynaecological medical device license
We have prepared this guide to help you easily navigate the CDSCO medical device license application process.
Documents required for securing CDSCO gynaecological medical device license
We have provided an overview of the documents required for securing CDSCO gynecological medical device license.
- Organization identity proof
- Sale Deed/Rent Deed of the Premises
- Plant Master File
- Building Layout with Dimension
- Device Master File
- Documents for the team of qualified and experienced staff who can manufacture and test your medical devices.
- Environmental regulation compliance documents
- ISO 13485 Certificate
- Certificate of analysis of 3 consecutive batches
- Test License, if required for testing the gynaecological device
For a detailed list of documents required, feel free to contact us. Additionally, we will help you prepare and compile the documents as per CDSCO guidelines. Our team has extensive expertise in document preparation for CDSCO medical device license application.
CDSCO gynaecological medical devices classification
To secure theCDSCO license for gynaecological medical devices, the first step is to understand the CDSCO medical device classification system. Medical devices are categorised based on the potential risks associated with their use. They are categorised into four classes, A, B, C, and D, in increasing order of risks. Notably, this classification system has been developed to simplify the license application process. Moreover, different licenses and application processes are required for the different CDSCO class.
Use our free tool to check which class your gynecological medical device belongs to. This will determine your CDSCO gynaecological medical device license application process.
CDSCO gynecological medical device classes
We have listed some examples of gynecological medical devices belonging to the different CDSCO classes.
Class A
- Abdominal decompression chamber
- Abdominal decompression chamber pump
- Birthing bed/table, powered
- Amniotic membrane perforator, reusable
- Birthing/delivery kit
- Heel stirrup
- Gynaecological operating table, electrohydraulic or electromechanical or hydraulic
CDSCO class B
- Abortion suction system manual aspirator
- Cervical anaesthesia kit
- Cervical anaesthesia needle, reusable or single use
- Endocervical aspirator
- Endocervical specimen collection kit, no
- additive
- Endometrial biopsy curette
- Endometrial biopsy kit
- Endometrial cytology brush
- Fallopian tube biopsy everting-balloon catheter
- Fallopian tube catheterization kit
- Fallopian tube insufflator
- Fixed-diameter cervical dilator, reusable or single use
- Flexible fibreoptic culdoscope
- Flexible fibreoptic hysteroscope
- Flexible fibreoptic laparoscope
- Flexible fibreoptic mammary ductoscope
- Flexible fibreoptic salpingoscope
- Flexible ultrasound laparoscope
- Flexible video culdoscope
- Flexible video hysteroscope
- Flexible video laparoscope
- Flexible video mammary ductoscope
- Flexible video salpingoscope
- Foetal Doppler system
Class C
- Cardiotocograph
- Cardiotocograph transducer
- Cardiotocography telemetric monitoring system
- Cardiotocography telemetric monitoring system receiver
- Cardiotocography telemetric monitoring system transmitter
- Colposcope
- Contraceptive cervical cap, reusable or single use
- Contraceptive spermicide
- Contraceptive sponge
- Diaphragm pessary
- Breast transilluminator
Class D
- Fallopian tube occlusion insert
Different CDSCO gynaecological medical device license types
Different licenses and application processes apply for the different CDSCO classes.
- Manufacturing class A and B gynaecological devices: You need to apply for MD 5 license. Moreover, a fee of Rs. 5,000 for the manufacturing license will be required. Additionally, Rs. 500 for each distinct device will be required. The license has to be obtained from the state licensing authority.
- Manufacturing class C and D gynaecological devices: You need to apply for MD 9 License. Moreover, a fee of Rs. 50,000 for the manufacturing license will be required. Additionally, Rs. 1,000 for each distinct device will be required. The license has to be obtained from the central licensing authority.
- Importing gynaecological devices into India: MD 15 Import License has to be secured from the CDSCO to import gynaecological medical devices into India.
Overview of the CDSCO gynaecological medical device license application process
The CDSCO medical device license application process involves the following steps:
- Check CDSCO class: The first step of the CDSCO medical device license application process is to check the CDSCO class. This will determine the license type to be used.
- Document preparation: Compile all necessary support documents required for license application.
- Application Submission: File the application online on the official CDSCO online portal. Ensure all details are accurate. Furthermore, properly attach all the documents.
- Query Resolution: CDSCO may raise queries that have to be promptly and accurately addressed. The queries can range from clarification of the submitted documents to requests for additional information.
- Inspection: Prepare for the CDSCO medical device facility inspection and audit phase. CDSCO officials may visit the facility to ensure regulatory compliance.
- License Approval: The regulatory officials will verify all the documents and review the application. Then, if all the criteria are satisfied, the CDSCO will grant the license. This license will permit you to manufacture or import, as applicable, gynecological medical devices.
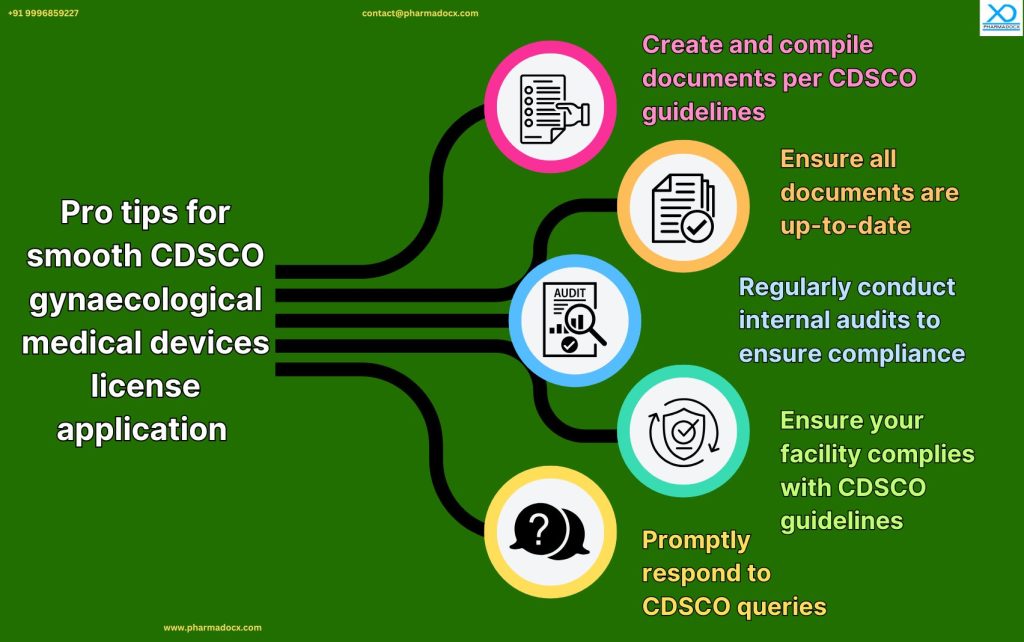
Common CDSCO medical device license application challenges and how to overcome them
Manufacturers and importers might face the following challenges while applying for their CDSCO medical device license. Hence, we have provided some suggestions on how to overcome them.
- Documentation Gaps: Ensure all documents are up-to-date and meet CDSCO’s guidelines.
- Delays in Query Resolution: Set up a dedicated team to promptly and accurately address CDSCO queries.
- Inspection Hurdles: Conduct regular internal reviews of the medical device facility to ensure they meet CDSCO guidelines and requirements.
Validity of the CDSCO medical device license
The CDSCO gynaecological medical device license is valid indefinitely. However, to maintain the validity, the license retention fee has to be paid every 5 years.
How can Pharmadocx Consultants assist you in securing the CDSCO medical devices license in a hassle-free manner?
The CDSCO medical device license application process is tricky and cumbersome. Extensive knowledge of the CDSCO guidelines will be required. Furthermore, applying for the license on the CDSCO portal is not an easy task. Additionally, numerous supporting documents will have to be prepared. Moreover, you have to accurately respond to the CDSCO queries. With the support of the Pharmadocx Consultants team, the CDSCO medical device licensing journey will be streamlined and efficient for you. We provide the following comprehensive services to medical device manufacturers to help them in their CDSCO license application journey.
- Assistance throughout the CDSCO medical device license application process.
- Document preparation and compilation as per CDSCO guidelines.
- Conducting pre-inspection audits to ensure readiness.
- Our team will help you promptly address CDSCO queries.
- Comprehensive support from the initial application to license approval. Our service does not end with CDSCO medical device license application. We will provide support till you successfully secure the CDSCO medical device license.
Are you planning to secure the CDSCO gynaecological medical device license? Drop an email at [email protected] or call/Whatsapp on 9996859227 to let us help you. Our team will ensure you have a seamless CDSCO medical device license application process.