CDSCO Manufacturing License for IVD Products Class C & D
Skip the confusion, delays, and rejections. Our team has guided over 300 medical device companies through CDSCO’s regulatory process for IVD Manufacturing, and we’re excited to do the same for yours.
Expert consultation with industry leaders
Fast & hassle-free approvals
Manufacturing Registration on Form MD9
Fast and accurate query replies
600+ Granted Approvals 27+ Years Experience
300+ Happy Clients












In vitro diagnostic devices (IVDs) are the cornerstone of modern healthcare. They can be used in patient’s homes, laboratories, or health care facilities. They play an indispensable role in diagnosis, treatment selection, and monitoring, thereby improving patient outcome by offering effective healthcare delivery. Hence, IVDs offer significant benefits for patient management. Therefore, IVDs entering the Indian markets have to be strictly regulated and monitored. Moreover, Class C and D IVDs are moderate to high-risk devices requiring stricter guidelines. Thus, you need to mandatorily secure the CDSCO license for manufacturing Class C and D IVDs to market your devices. Planning to manufacture IVDs in India? Need help securing the CDSCO IVD manufacturing license? We have got you covered.
CDSCO IVD regulations are difficult to comprehend. Moreover, a knowledge of the CDSCO IVD classification system is required while applying for CDSCO IVD manufacturing license. We at Pharmadocx Consultants provide comprehensive regulatory support to IVD manufacturers in India. Our team of experts will help you easily navigate the CDSCO regulatory guidelines for manufacturing IVDs in India.
CDSCO Regulations for Manufacturing IVDs in India
CDSCO has formulated IVD regulations to provide guidelines for manufacturing safe, effective, and high-quality IVDs. Medical Device Rules, 2017, and Good Manufacturing Practices provide the regulatory framework for manufacturing IVDs in India. IVD manufacturers are expected to comply with these regulations issued by CDSCO. Companies intending to manufacture IVDs in India need to mandatorily secure the CDSCO IVD manufacturing license. Different regulatory guidelines and license types are applicable for different CDSCO IVD classes. IVD manufacturers have to apply for their CDSCO IVD license accordingly. We will walk you through the CDSCO IVD classification system and CDSCO IVD manufacturing license application process.
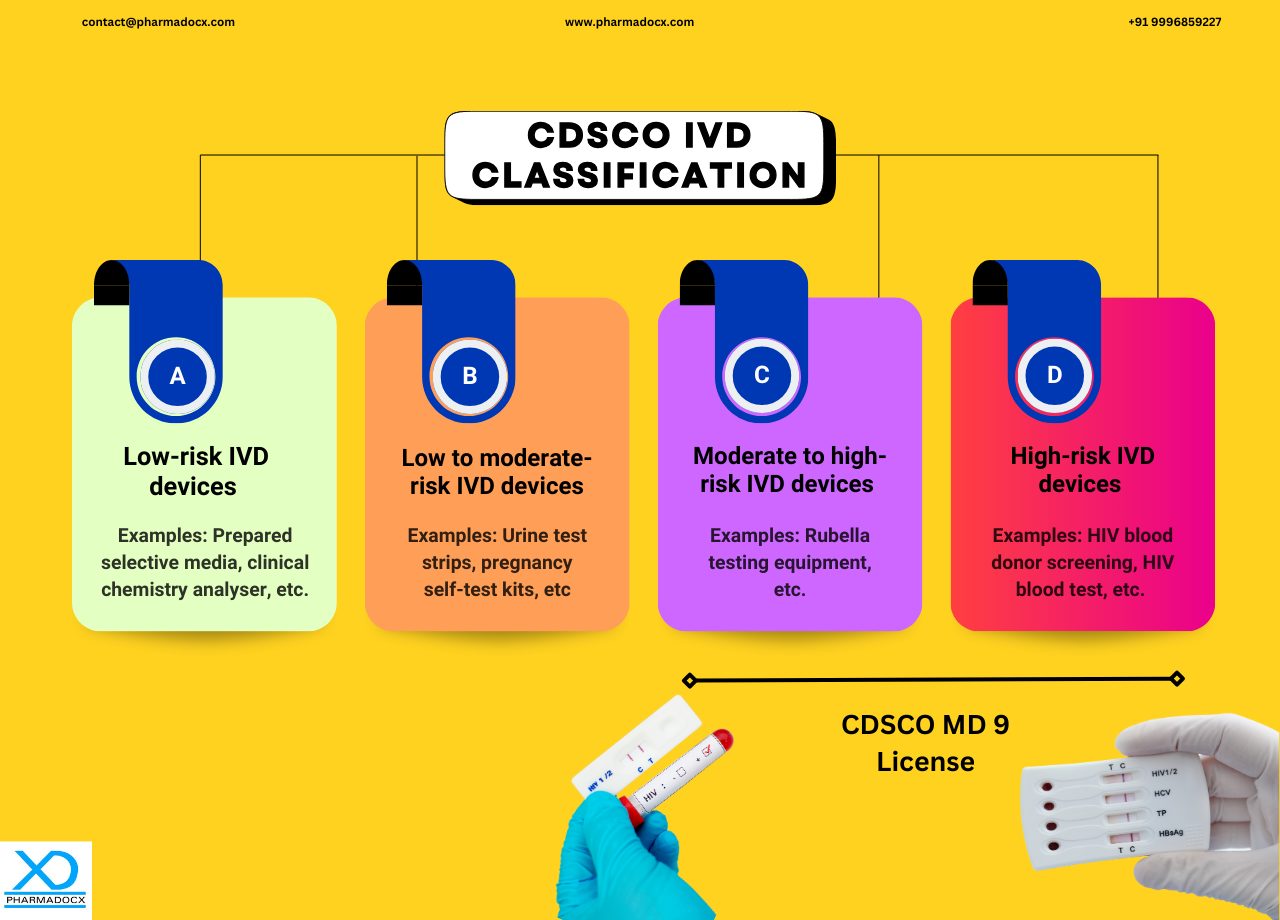
CDSCO IVD Classification System
The CDSCO IVD classification system is based on the intended use and risk associated with the device. IVDs have been categorised into 4 classes, A, B, C, and D.
What are Class C and D IVDs?
CDSCO class C IVDs
CDSCO class C IVDs are moderate to high risk IVD devices having the following intended use:
- Pre-natal screening of women to analyse their immune system status with respect to transmissible agents.
- IVD device to be used for detecting the presence of an infectious agent, in case there is a significant risk that an incorrect result will be detrimental.
- IVD device to be used for detecting infective disease status or immune system status, where there is a risk that an incorrect result will lead to an imminent life-threatening situation for the patient being tested.
- In vitro diagnostic devices intended for testing human genetics.
- Devices to be used for blood gas analysis or a blood glucose determination.
- Devices used for screening of disease staging in order to select patients for selective therapy and management.
- Monitoring levels of biological or substance components or medicinal items, where there is a risk that an erroneous result will be life-threatening.
- In vitro diagnostic devices intended for managing patients suffering from life-threatening infectious diseases.
- IVD device intended for detecting the presence or exposure to a sexually transmitted disease.
- In vitro diagnostic devices intended for detecting the presence of an infectious agent in the cerebrospinal fluid/blood.
- IVDs intended for blood grouping or tissue typing. Additionally, devices intended to evaluate the immunological compatibility of any cell, tissue, blood and blood component, blood derivative, or organ that is intended for transplantation or transfusion.
- IVD devices for screening of congenital disorders in the foetus.
Examples: Hepatitis testing kits, HIV testing kits, cancer diagnostic kits, etc.
Looking for CDSCO IVD manufacturing license?
Simply fill out the form below and we will get back to you
CDSCO class D IVD devices
CDSCO class D IVDs are highest risk IVD devices having the following intended use:
- IVD devices for detecting the presence of or exposure to a transmissible agent.
- IVD devices for detecting any cell, tissue, blood, blood component, blood derivative, or organ for transplantation or transfusion. Additionally, in vitro diagnostic devices for detecting the aforementioned components that can cause a life-threatening disease with a high risk of propagation.
Examples: Companion diagnostics and some laboratory-developed tests
CDSCO License for Manufacturing Class C and D IVDs
Manufacturing license
For manufacturing Class C and D IVDs in India, a MD 9 license will be required. The applicant will have to use MD 7 to apply for a license to manufacture, sell, and distribute these IVD devices in India. The license to manufacture Class C and D IVD devices for sale and distribution in India will be granted under MD 9.
Loan license
A loan license permits a company without a factory and facility to manufacture IVDs. With the loan license, the company can use the manufacturing facility of another company that is manufacturing the same IVD. The applicant will have to use MD 8 to apply for a loan license for manufacturing Class C and D IVD devices. Loan license to manufacture these IVDs without a facility and factory will be granted under MD 10.
Documents required for securing the CDSCO License for Manufacturing Class C and D IVDs
- Constitution proof (GST Certificate / CIN / MOA AOA)
- Cover letter
- Organization identity proof, such as UDYAM Aadhar, PAN card, etc.
- Sale Deed/Rent Deed proving legal ownership of the manufacturing facility.
- Plant master file
- Device master file
- ISO 13485Certificate
- Certificate of analysis of 3 consecutive batches
- Fire and Pollution NOCs
- Post marketing surveillance data
- Manufacturing facility building layoutpresenting the dimensions of each room and the location of all the equipment in the facility.
- Documents proving the manufacturing team comprises competent, qualified, and experienced staff.
- Test license (if applicable)
- In case of loan license application, consent letter from the principal manufacturing unit, principal manufacturer’s license and product permit, and wholesale license of the applicant.
To avail our comprehensive document preparation service, feel free to reach out to us. Our team will prepare all the necessary supporting documents per CDSCO guidelines. We will ensure the documents align perfectly with CDSCO requirements, increasing chances of swift CDSCO IVD manufacturing license approval.
Securing MD9 License for Manufacturing Class C and D IVDs: Step-by-Step Guide
Step 1 – Applicant Registration
- Portal Login: Sign in or login to CDSCO online registration portal.
Step 2 – MD9 License Application for Manufacturing Class C and D IVDs
- Form Filling: Fill the MD7 application form. Details, such as device’s classification, brand name, intended use, and a detailed product description, will be required.
- Document Submission: Prepare and upload essential supporting documents required for license application. This will include ISO 13485 certificate, Plant Master File, Device Master File, organisation details etc.
- Fee Payment: Pay the necessary CDSCO license application fee, which will depend on the device’s classification.
- Application Confirmation: Once all documents are submitted and fees are paid, you will receive an application number. This will confirm successful submission.
Step 3 – CDSCO will Review the Application
- Application Review: CDSCO will meticulously review the application and all attached documents.
- CDSCO Queries: If there are queries or discrepancies, CDSCO will reach out for clarification or additional information.
- Query Response: CDSCO queries have to be promptly and accurately addressed. Submit any requested justifications or revised documents through the portal.
Step 4 – CDSCO Audit
- Facility Audit and Inspection: CDSCO officials will audit your factory for regulatory compliance.
Step 5 – License Approval for Manufacturing Class C and D IVDs
- Final Review: CDSCO will once again review and comment on the justifications and documents. If everything aligns with the regulations, the officials will move forward with the application.
- License Grant: Once all criteria are satisfied and queries are responded, the MD9 manufacturing license will be granted.
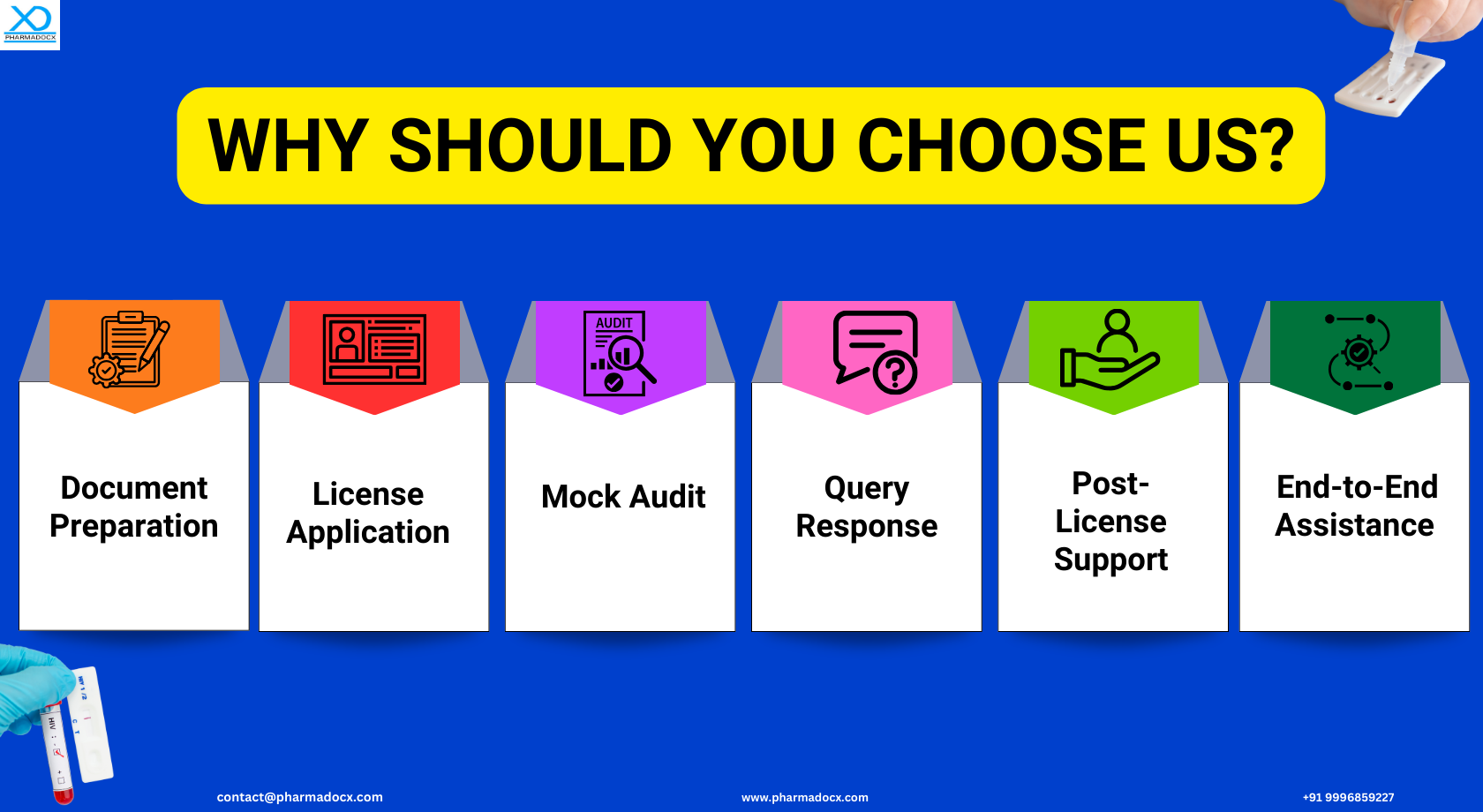
Why Should You Choose Pharmadocx Consultants as Your Regulatory Partner?
Our team is well-versed with the nuances of CDSCO guidelines for IVDs. We will ensure your application aligns perfectly with the CDSCO regulatory requirements, thereby increasing chances of swift license grant.
At Pharmadocx Consultants, our clients are our top priority. We tailor our services to meet your specific needs, ensuring a personalized experience. Backed by years of experience, our license application process is streamlined and time-efficient. We offer cost-effective comprehensive client-centric services to IVD manufacturers. Call/Whatsapp us at 9996859227 or write to us at [email protected] for easily securing MD 9 license for manufacturing class C and D IVDs.
Let's Talk!
We'd love to hear from you! Whether you have questions about our pharmaceutical plant setup consultation services or want to discuss a potential project, our team is here to help. Simply fill out the form below, and we'll get back to you as soon as possible. Alternatively, you can reach out to us directly using the phone number or email address listed on this page. We look forward to connecting with you!
Phone / Whatsapp
Address
- Head Office - Opposite Dewan Mill, Old D.C. Road Sonepat - 131001 Haryana, India
- Registered Office - Netaji Subhash Place, Delhi, 110034