CDSCO Medical Device License & Registration
Easily secure CDSCO License in the shortest time and least hurdles.
Skip the confusion, delays, and rejections. Our team has guided over 300 medical device companies through CDSCO’s regulatory process, and we’re excited to do the same for yours.
Fast & hassle-free approvals
Manufacturing & Import Registration
Documentation that passes scrutiny the first time
Fast and accurate query replies
600+ Granted Approvals 27+ Years Experience
300+ Happy Clients












What is CDSCO Medical Device Registration?
CDSCO Registration refers to getting regulatory clearance from Central Drugs Standard Control Organization for manufacturing, import & sale purpose. CDSCO MD Online is the official portal where all registrations, approvals and licensing is done.
Central Drugs Standard Control Organization (CDSCO) is the apex regulatory body for all medical devices in India. It has formulated stringent guidelines for all medical devices to prevent substandard medical devices from entering the Indian market. Hence, securing the CDSCO license for medical devices is a mandatory requirement.
Before getting Medical Device Registration, it’s important to classify your medical device as per CDSCO’s notified lists.
CDSCO Medical Device Classification
For ease of medical device registration and licensing, the CDSCO has introduced a risk-based medical device classification system. Medical devices have been categorised into four classes, A, B, C, and D, based on the risk-level associated with their intended use. Check Medical Device CDSCO Class here.
Class A
Low-risk medical devices that pose minimal or no potential risk to patients or users
Class B
Low-to-moderate-risk medical devices that may cause harm to patients or users, if they malfunction. However, they are not life-threatening.
Class C
Moderate-to-high-risk medical devices that have the potential to cause serious harm or injury to patients, if they malfunction.
Class D
High-risk medical devices that are critical to the health and survival of patients. If they malfunction, it could lead to serious harm or death
CDSCO Manufacturing License Registration for Medical Devices
There are primarily 4 types of CDSCO Registrations for purpose of manufacturing medical devices in India. Each type depends on the classification of medical device being manufactured and the site being used for manufucturing. These are –
CDSCO License for manufacturing Class A & B Medical Devices
MD 5 license is required to manufacture class A & B medical devices. A fee of Rs. 5,000 for the manufacturing license and Rs. 500 for each distinct device is required. The license will be issued by the State Licensing Authority.
CDSCO License for manufacturing Class C & D Medical Devices
MD 9 license is required to manufacture class C & D medical devices. A fee of Rs. 50,000 for the manufacturing license and Rs. 1,000 for each distinct device is required. The license will be issued by the Central Licensing Authority.
CDSCO Test License for Medical Devices
CDSCO grants the test license for manufacturing small quantities of medical devices for testing, clinical research, and demonstration purposes. CDSCO will grant the test license for manufacturing medical devices in India under MD-13.
CDSCO Loan License for Medical Devices
Manufacturers who do not have a medical device manufacturing facility can apply for the CDSCO loan license. These manufacturers will have to manufacture their devices in the facility of another medical device manufacturer. The loan license will be granted provided the other manufacturer manufactures the same product as the licensee.
| Class of Medical Device | Application Form | License Required | Application Fee | Avg Time |
|---|---|---|---|---|
| Class A (Non Sterile Non Measuring) | - | Class A Registration | Free | 1 Day |
| Class A (Sterile/Measuring) | Form MD3 | MD5 License | Rs 5,000 per site, Rs 500 per product | 2-3 Months |
| Class B | Form MD3 | MD5 License | Rs 5,000 per site, Rs 500 per product | 2-3 Months |
| Class C | Form MD7 | MD9 License | Rs 50,000 per site, Rs 1,000 per product | 3-4 Months |
| Class D | Form MD7 | MD9 License | Rs 50,000 per site, Rs 1,000 per product | 3-4 Months |
CDSCO Import License Registration for Medical Devices
For importing medical devices into India, getting the CDSCO MD15 medical device import license is a mandatory requirement. MD 15 license is required to import medical devices into India. The license will be issued by the Central Licensing Authority. MD15 License process takes an average of 5 months.
Exception: For Class A Non Sterile Non Measuring Devices, MD15 license is not required. Instead, Class A Registration for Import is required.
Regulations for Bringing Raw Materials, Semi-Finished Products, or Components into India
In certain cases, medical device companies have to bring raw materials, semi-finished products, or components into India. If the final assembly and packaging of the medical device is done in India with these materials, a CDSCO manufacturing license will be required. However, if the complete finished product is brought in from outside India, a CDSCO import license will be required.
| Class of Medical Device | Application Form | License Required | Application Fee | Avg Time |
|---|---|---|---|---|
| Class A (Non Sterile Non Measuring) | - | Class A Registration | Free | 1 Day |
| Class A (Sterile/Measuring) | Form MD14 | MD15 License | $1,000 per site, $50 per product | 5 Months |
| Class B | Form MD14 | MD15 License | $2,000 per site, $1000 per product | 5 Months |
| Class C | Form MD14 | MD15 License | $3,000 per site, $1,500 per product | 5 Months |
| Class D | Form MD14 | MD15 License | $3,000 per site, $1,500 per product | 5 Months |
Who can apply for CDSCO Registration in India?
The following entities can apply for CDSCO medical device registration:
- Domestic Medical Device Manufacturers
- Foreign Medical Device Manufacturers
- Medical Device Importers
- Authorised agent for Medical Devices and IVDs
- Indian subsidiary of medical device companies
Looking for CDSCO Registration?
Documents required for CDSCO Medical Device Registration in India
For Manufacturing License
- Covering Letter
- Company Constitution
- Premises Ownership Proof
- Plant Master File
- Device master file
- Essential Principles Checklist
- Risk Management File
- ISO 13485 (for quality management system)
- Inspection / Audit Report
- Payment Receipt (Challan)
- Layout of premises
- Test License on Form MD13
Import License
- Covering Letter
- Power of Attorney notorised by first class magistrate of India or appostiled
- Company Constitution
- Premises Ownership Proof
- Plant Master File
- Device master file from foreign manufacturer
- Free sale certificate from the country of origin is required. This is required to certify the product is freely sold and legally marketed in its country of origin.
- Essential Principles Checklist
- Risk Management File
- ISO 13485 (for quality management system)
- Payment Receipt (Challan)
We have provided an overview of the documents required to obtain a CDSCO medical device license. For a detailed list of documents required, feel free to get in touch with us. Furthermore, we provide document preparation support. Our team will not only prepare the documents but also compile them per CDSCO regulatory guidelines.
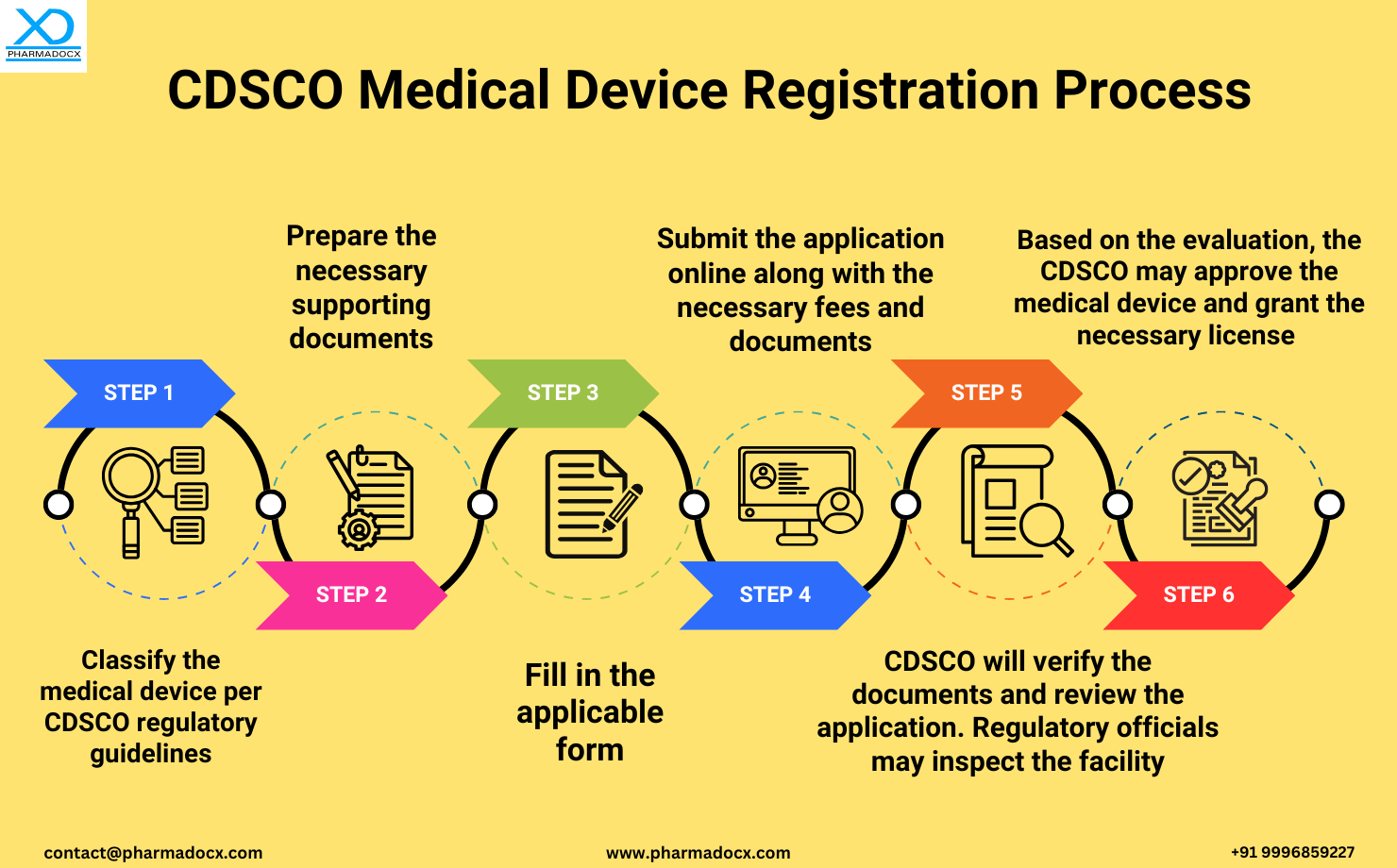
Step-by-step Guide to the Process of CDSCO Medical Device Registration in India
Registering a medical device with the Central Drugs Standard Control Organization (CDSCO) is a critical step to ensure compliance with Indian regulatory standards. The process involves the following key steps:
- Step 1: Device Classification
The first step is to classify the medical device according to CDSCO regulatory guidelines. Accurate classification determines the applicable regulatory pathway. - Step 2: Documentation Preparation
Prepare all necessary supporting documents, including technical details, safety data, and quality certifications relevant to the device. - Step 3: Application Form Completion
Fill out the applicable CDSCO registration form with all required information, ensuring accuracy and completeness. - Step 4: Online Submission
Submit the completed application online along with the necessary fees and documents via the CDSCO portal. - Step 5: Review and Inspection
CDSCO officials will verify the submitted documents and review the application. If necessary, a facility inspection may be conducted. - Step 6: Approval and Licensing
Upon successful evaluation, CDSCO will approve the medical device and issue the required license, authorizing its legal sale and distribution in India.
How can Pharmadocx Consultants help you get CDSCO Registration?
- Regulatory support: We have extensive knowledge of the CDSCO regulatory guidelines and industry requirements. We will help you navigate the complex regulatory landscape and identify the guidelines applicable for your medical device.
- Assistance with classification of medical devices: Different CDSCO classes of medical devices have different regulatory requirements. We will help you identify the correct class for your medical device and ensure compliance with applicable requirements.
- Comprehensive documentation support: We provide holistic CDSCO document preparation and compilation support.
- Online application submission support: We help our clients navigate the online application submission process. Additionally, we help them submit their applications on the official CDSCO application portal.
- Mock audits: We conduct mock audits to identify gaps and provide actionable next steps to correct the lapse. Additionally, we will train your staff and prepare them for the regulatory audit.
- Communication liaison with CDSCO: We act as a liaison between clients and the CDSCO. We promptly handle and respond to the CDSCO queries on behalf of the clients.
- Quality management system: We offer guidance on establishing a robust quality management system as per CDSCO requirements.
- Post-license application support: Our service does not end with license application. We provide assistance till you successfully secure the CDSCO medical device license. Additionally, we provide support for license renewals and compliance with updated regulatory guidelines.
Want to easily secure the CDSCO medical device registration? For a smooth CDSCO regulatory journey, call/Whatsapp on 9996859227 or email at [email protected]. We will streamline the process and increase the chances of successful registration of your device with CDSCO.
Let's Talk!
We'd love to hear from you! Whether you have questions about our pharmaceutical plant setup consultation services or want to discuss a potential project, our team is here to help. Simply fill out the form below, and we'll get back to you as soon as possible. Alternatively, you can reach out to us directly using the phone number or email address listed on this page. We look forward to connecting with you!
Phone / Whatsapp
Address
- Head Office - Opposite Dewan Mill, Old D.C. Road Sonepat - 131001 Haryana, India
- Registered Office - Netaji Subhash Place, Delhi, 110034
CDSCO Medical Device Registration for Different Notified Categories
- CDSCO License for Anesthesiology Medical Devices
- CDSCO License for Cardiovascular Medical Devices
- CDSCO License for Dental Medical Devices
- CDSCO License for Dermatological and Plastic Surgery Medical Devices
- CDSCO License for ENT Medical Devices
- CDSCO License for Gastroenterology Medical Devices
- CDSCO License for General Hospital Medical Devices
- CDSCO License for Interventional Radiology Medical Devices
- CDSCO License for In-Vitro Diagnostic Medical Devices
- CDSCO License for Nephrology Medical Devices
- CDSCO License for Neurological Medical Devices
- CDSCO License for Obstetrical and Gynecology Medical Devices
- CDSCO License for Oncology Medical Devices
- CDSCO License for Operation Theatre Medical Devices
- CDSCO License for Ophthalmology Medical Devices
- CDSCO License for Orthopaedic Medical Devices
- CDSCO License for Pain Management Medical Devices
- CDSCO License for Pediatrics and Neonatology Medical Devices
- CDSCO License for Personal Protective Equipment Medical Devices
- CDSCO License for Physical Support Medical Devices
- CDSCO License for Radiotherapy Medical Devices
- CDSCO License for Rehabilitation Medical Devices
- CDSCO License for Respiratory Medical Devices
- CDSCO License for Software as Medical Devices (SaMD)
- CDSCO License for Surgical Instruments for General Use Medical Devices
- CDSCO License for Urology Medical Devices
Frequently Asked Questions (FAQs)
Which license is required for manufacturing Class A Medical Devices?
For Non Sterile Non Measuring Class A Medical Devices – CDSCO Class A Registration is required
For Sterile or Measuring Class A Medical Devices – MD5 License is required
Which license is required for manufacturing Class B Medical Devices?
CDSCO MD5 License is required for Class B Medical Devices.
Which license is required for manufacturing Class C Medical Devices?
CDSCO MD9 License is required for manufacturing Class C Medical Devices.
Which license is required for manufacturing Class D Medical Devices?
CDSCO MD9 License is required for manufacturing Class D Medical Devices.
Which license is required to import Class A Medical Devices?
For Non Sterile Non Measuring Devices – CDSCO Class A registration is required
For Sterile or Measuring Class A Devices – MD15 License is required
Which license is required to import Class B, C & D Medical Devices?
CDSCO MD15 License is required to import Class B, C & D Medical Devices
Do I need CDSCO license for Class A Non-sterile Non-measuring medical devices?
No, only CDSCO Class A registration is required to manufacture or import Class A Non Sterile Non Meassuring Medical Devices
What is government fee for CDSCO MD5 License?
The government fees for MD5 License is –
- Rs 5000 per application
- Rs 500 per product
What is government fee for CDSCO MD9 License?
The government fees for MD5 License is –
- Rs 50,000 per application
- Rs 1,000 per product
What is government fee for CDSCO MD15 import License?
The government fees for CDSCO MD15 Import License is –
- Class A Sterile/Measuring Products – $1,000 per site & $50 per product
- Class B Medical Devices – $2,000 per site & $1,000 per product
- Class C & D Medical Devices – $3,000 per site & $1,500 per product

