Diabetes management devices are at the forefront of revolutionizing care for diabetic patients. With the rise in prevenance of diabetes in India, patients need to access high-quality devices. Hence, the Central Drugs Standard Control Organization (CDSCO), the apex regulatory body, has set quality benchmarks for diabetes management devices. Manufacturers and importers of these devices are required to comply with these regulatory guidelines. This is a comprehensive guide on securing the CDSCO license for manufacturing or importing diabetes management medical devices. Additionally, we have explained the CDSCO classification system for diabetes medical devices. Furthermore, we have provides some tips on how to overcome the common CDSCO medical device license application hurdles.
Understanding CDSCO Classification System for Diabetes Management Medical Devices
Medical devices are classified based on the potential risks associated with their use. They are categorised into four classes, A, B, C, and D. Notably, CDSCO class A, B, C, and D devices are associated with increasing order of risk levels. Additionally, different license application process and forms are required for the different CDSCO classes.
Diabetes management medical devices are essential in monitoring and managing blood glucose levels effectively. Hence, given their important role in the healthcare sector, the quality, efficacy, and safety of these devices have to be stringently regulated. Furthermore, it is important to understand the CDSCO classification system for diabetes management devices to proceed with the license application process. Thus, to check which class your diabetes management device belongs to, you can use our free tool.
Diabetes Management Medical Device Classes
We have provided few examples of diabetes management medical devices belonging to each CDSCO class.
CDSCO class B:
- Insulin syringes
Class C:
- Diabetes management test/kit
- Insulin pens
- Glucose monitoring systems
- Diabetic foot ulcers treatment devices
CDSCO class D:
- Insulin pumps
- Continuous glucose monitoring systems (CGMS)
- Artificial pancreas devices
Securing CDSCO License for Diabetes Management Medical Devices
You have to secure a CDSCO license to manufacture or import diabetes medical devices in India. We have listed the different types of licenses. Additionally, we have provided an overview of the documents required. Furthermore, we have provided a step-by-step guide of the CDSCO license application process. However, the CDSCO guidelines can be difficult to comprehend and the license application process can be overwhelming. Fret not! Feel free to contact us for a smooth and hassle-free CDSCO medical device regulatory journey.
CDSCO License Types for Diabetes Management Medical Devices
- Manufacturing class A and B diabetes management devices: MD 5 License will be required to manufacture class A and B diabetes management devices. Moreover, a fee of Rs. 5,000 for the manufacturing license will be required. Additionally, Rs. 500 for each distinct device is required. The license has to be obtained from the state licensing authority.
- Manufacturing class C and D diabetes management devices: MD 9 License will be required to manufacture class C and D diabetes management devices.Moreover, a fee of Rs. 50,000 for the manufacturing license will be required. Additionally, Rs. 1,000 for each distinct device is required. The license has to be obtained from the central licensing authority.
- Importing diabetes management devices into India: MD 15 Import License has to be secured from the CDSCO to import diabetes management devices into India.
Documents Required for CDSCO Diabetes Management Medical Devices License Application
We have provided an overview of the documents required for securing the CDSCO license.
- Organization identity proof
- Sale Deed/Rent Deed of the Premises
- Plant Master File
- Building Layout with Dimension
- Device Master File
- Documents for the team of qualified and experienced staff who can manufacture and test your medical devices.
- Test License, if required for testing the diabetes management device
- Environmental regulation compliance documents
- Certificate of analysis of 3 consecutive batches
- ISO 13485 Certificate
A Step-by-step Guide for CDSCO Diabetes Management Medical Devices License
The CDSCO registration pathway for diabetes management devices involves:
- Documentation: Compile all necessary documents, including clinical trial data and product specifications.
- Application Submission: File the application online.
- Query Resolution: CDSCO may raise queries that have to be promptly and accurately addressed.
- Inspection: Prepare for the CDSCO inspection phase.
- License Approval: Upon meeting all criteria, CDSCO will grant the license.
Validity of the CDSCO Diabetes Management Medical Devices License
The CDSCO diabetes management device license is valid indefinitely. However, to maintain the validity, the license retention fee has to be paid every 5 years.
The New CDSCO Guidelines: What’s Changed?
CDSCO, in its commitment to patient safety and product excellence, has updated its guidelines for diabetes management medical devices. For Class C and Class D diabetes management devices, the emphasis is on rigorous testing and quality assurance. Furthermore, the manufacturing process has to align with international best practices and guidelines. Thus, CDSCO has updated its guidelines to ensure medical devices manufactured in India are at par with those manufactured internationally.
The Deadline: Key Dates to Remember
CDSCO has transitioned Class C and Class D diabetes management devices from the mandatory registration protocol to a licensing structure. This has been detailed in GSR 102(E) dated 11.02.2020. Notably, this significant transition will be in effect from October 1, 2023.
Manufacturers and importers are advised to update themselves with the Medical Devices Rules (MDR) 2017. They are required to submit their applications through CDSCO’s online portal, complete with the required documents and fees. For a more in-depth understanding, the official CDSCO circular is a go-to resource.
Common CDSCO License Application Challenges and How to Overcome Them
Manufacturers and importers might face the following challenges. Additionally, we have provided some tips on how to tackle these challenges.
- Documentation Gaps: Ensuring all documents are current and resonate with CDSCO’s standards.
- Delays in Query Resolution: Establishing a dedicated team to address CDSCO queries swiftly.
- Inspection Hurdles: Conducting regular internal audits of facilities to ensure they align with CDSCO guidelines.
How Can Pharmadocx Consultants Assist in Navigating CDSCO Diabetes Management Medical Devices License Application?
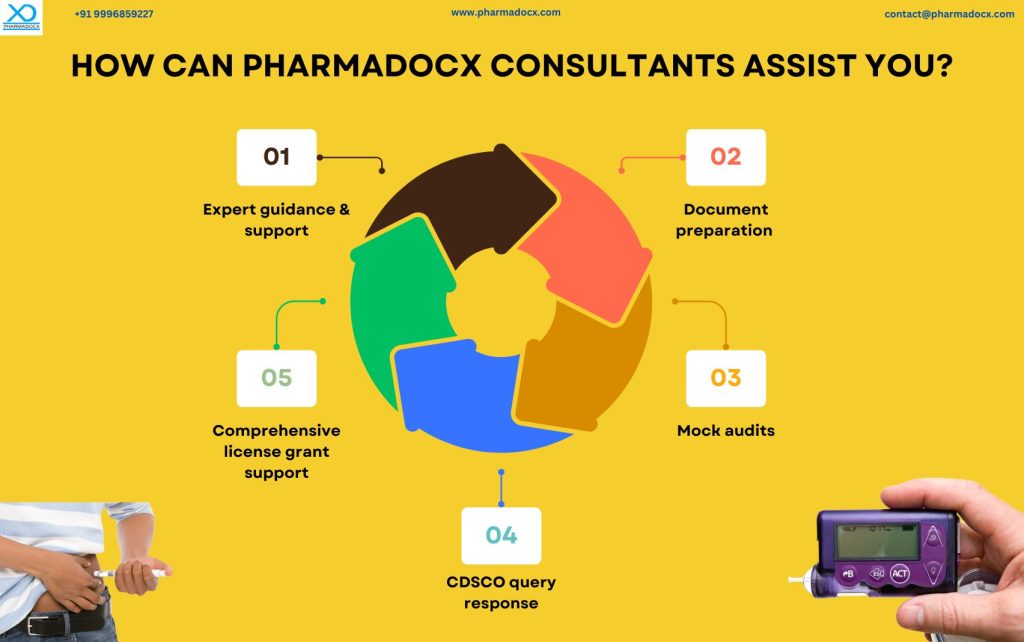
Regulatory compliance is a testament of the manufacturer’s commitment to patient safety and product quality. Additionally, ensuring compliance not only fosters trust but also solidifies the brand’s reputation in the market. Hence, having the CDCSO medical device license is not just a regulatory commitment, it is beneficial for the manufacturer. We at Pharmadocx Consultants offer a suite of services tailored for diabetes management device manufacturers to help simplify their regulatory journey:
- Expert Guidance: Assistance throughout the CDSCO medical device registration process.
- Mock Audits: Conducting pre-inspection audits to ensure readiness.
- Query Resolution: Our team will help you promptly address CDSCO queries.
- End-to-End Support: Comprehensive support from the initial application to license approval. We will provide support till you successfully secure the CDSCO medical device license.
- Learn More
CDSCO registration for diabetes management devices ensures that healthcare professionals are equipped with the most advanced tools for diabetes care. The CDSCO diabetes management medical devices license application is a tricky task. Extensive knowledge of the CDSCO regulations and expertise in CDSCO license application is required. It is not possible for diabetes management device manufacturers to constantly stay abreast of the latest CDSCO regulations. With the support of expert partners like Pharmadocx Consultants, the CDSCO registration pathway will become a cake walk. Drop an email at [email protected] or call/Whatsapp on 9996859227 to get expert guidance and support for the CDSCO licensing process.





