Orthopedic and prosthetic devices are indispensable in the medical world, aiding countless individuals in mobility and improving their quality of life. These devices play a pivotal role in surgeries, rehabilitation, and enhancing mobility. The Central Drugs Standard Control Organization (CDSCO) ensuring the safety and efficacy of these devices in India. Hence, it’s imperative for manufacturers and importers to be well-versed with the process of CDSCO registration for orthopedic medical devices. This guide sheds light on the CDSCO registration for orthopedic and prosthetics devices and the associated regulatory framework.
CDSCO Registration for Orthopedic Medical Devices
Understanding Medical Device Classification for CDSCO Registration for Orthopedic Medical Devices
To understand the process of CDSCO registration for orthopedic medical devices, first let us understand CDSCO medical device classification system. Medical devices are classified based on the potential risks associated with their use. Similar to other medical devices, orthopedic and prosthetics devices have also been classified into the four CDSCO classes. We have listed some examples of orthopedic medical devices belonging to the different classes.
Orthopedic medical device classes
This medical device classification system helps simplify the CDSCO registration for orthopedic medical devices. Orthopedic and prosthetics devices mainly belong to B, C, and D classes. Use our free tool to check the class to which your medical device belongs to.
Class B orthopedic medical devices:
- Intra Osseous Fixation Wire
- Bone Wire
- Bone cap
- Plates, Clipers Screws
Class C orthopedic medical devices:
- Cortical Fixation Implant / rigidloop Adjustable Cortical Fixation System
- Intervertebral Body Fusion Device / Fuse Spinal System
- Orthopedic implant & accessories
- Pedicle screw spinal system
- Ankle joint metal/composite semi-constrained cemented prosthesis
- Ankle joint metal/polymer non-constrained cemented prosthesis
- Elbow joint humeral (hemi- elbow) metallic uncemented prosthesis
- elbow joint humeral (hemi- elbow) metallic uncemented prosthesis
- Finger joint metal/metal constrained uncemented prosthesis
- Finger joint polymer constrained prosthesis
- hip joint metal/composite semi-constrained cemented prosthesis
- Hip joint metal/ceramic/poly mer semi- constrained cemented or nonporous uncemented prosthesis
- Hip joint metal/polymer/met al semi-constrained porous-coated uncemented prosthesis.
- A knee joint femorotibial metallic constrained cemented prosthesis is a device intended to be implanted to replace part of a knee joint
- Shoulder joint metal/metal or metal/polymer constrained cemented prosthesis
- Wrist joint carpal lunate polymer prosthesis
- Wrist joint carpal scaphoid polymer prosthesis
- Smooth or threaded metallic bone fixation fastener
- Sacroiliac joint fixation
- Resorbable calcium salt bone void filler device
- Spinal interlaminal fixation orthosis
- Spinal intervertebral body fixation orthosis
- Toe joint polymer constrained prosthesis
- Shoulder joint humeral (hemi- shoulder) metallic uncemented prosthesis.
- knee joint patellofemorotibial metal/polymer
- Knee joint patellofemorotibial polymer/metal/pol ymer semi-constrained cemented prosthesis.
- Knee joint patellofemorotibial polymer/metal/met al constrained cemented prosthesis
- knee joint patellofemoral polymer/metal semi-constrained cemented prosthesis
- Knee joint femorotibial (uni- compartmental) metal/polymer porous coated uncemented prosthesis
- Knee joint femorotibial metal/polymer semi-constrained cemented prosthesis
- Knee joint femorotibial metal/polymer non-constrained cemented prosthesis
- Knee joint femorotibial metal/composite non-constrained cemented prosthesis
- Hip joint metal/polymer or ceramic/polymer semiconstrained resurfacing cemented prosthesis.
Examples of Class D orthopedic medical devices:
- Hip joint metal/metal semi-constrained, with a cemented acetabular component, prosthesis
- hip joint metal constrained cemented or uncemented prosthesis
- Intervertebral body fusion device
- Cervical Artificial Disc
- Hip joint metal/polymer constrained cemented or uncemented prosthesis
- Hip joint femoral (hemi-hip) metallic resurfacing prosthesis
- Hip joint femoral (hemi-hip) trunnion-bearing metal/polyacetal cemented prosthesis.
- A hip joint (hemi- hip) acetabular metal cemented prosthesis
- Hip joint femoral (hemi-hip) metallic cemented or uncemented prosthesis.
CDSCO Registration for Orthopedic Medical Devices in India: Licensing and Requirements
- Class A and Class B orthopedic devices: MD 5 License is required. A fee of Rs. 5,000 for the manufacturing license and Rs. 500 for each distinct device is required. The license has to be obtained from the state licensing authority.
- Class C and D orthopedic medical devices: MD 9 License is required.A fee of Rs. 50,000 for the manufacturing license and Rs. 1,000 for each distinct device is required. The license has to be obtained from the central licensing authority.
- Importing orthopedic medical devices into India: MD 15 Import License has to be secured from the CDSCO to import orthopedic medical devices into India.
New Guidelines for CDSCO Registration for Orthopedic Medical Devices: What’s Changed?
CDSCO, committed to patient safety and product quality, has refined its guidelines over time. For Class C & Class D orthopedic and prosthetics devices, the emphasis is on rigorous testing, quality checks, and alignment with international standards. Hence, it is important to constantly update oneself with the latest guidelines for CDSCO registration for orthopedic medical devices.
The Deadline: Key Dates to Remember for CDSCO Registration for Orthopedic Medical Devices
CDSCO has transitioned Class C and Class D orthopedic and prosthetics devices from mandatory registration system to a licensing structure. This has been detailed in GSR 102(E) dated 11.02.2020. Notably, this change will be in effect from October 1, 2023.
Manufacturers and importers are advised to take note of the Medical Devices Rules (MDR) 2017. They should keep in mind the guidelines while submitting their applications through CDSCO’s online portal. Moreover, for an in-depth understanding, the official CDSCO circular serves as a comprehensive resource.
Documents Required for CDSCO Registration for Orthopedic Medical Devices in India
We have provided an overview of the documents required for securing CDSCO orthopedic medical device registration and license.
- Organization identity proof
- Sale Deed/Rent Deed of the Premises
- Plant Master File
- Building Layout with Dimension
- Device Master File
- Documents for the team of qualified and experienced staff who can manufacture and test your medical devices.
- Test License, if required for testing the orthopedic medical device
- Environmental regulation compliance documents
- Certificate of analysis of 3 consecutive batches
- ISO 13485 Certificate
A Simplified Overview of the Process for CDSCO Registration for Orthopedic Medical Devices in India
The CDSCO registration process for orthopedic and prosthetic devices involves:
- Documentation: Compile all necessary documents, including clinical trial data and product specifications.
- Application Submission: File the application online.
- Query Resolution: CDSCO may raise queries that have to be promptly and accurately addressed.
- Inspection: Prepare for the CDSCO inspection phase.
- License Approval: Upon meeting all criteria, CDSCO will grant the license.
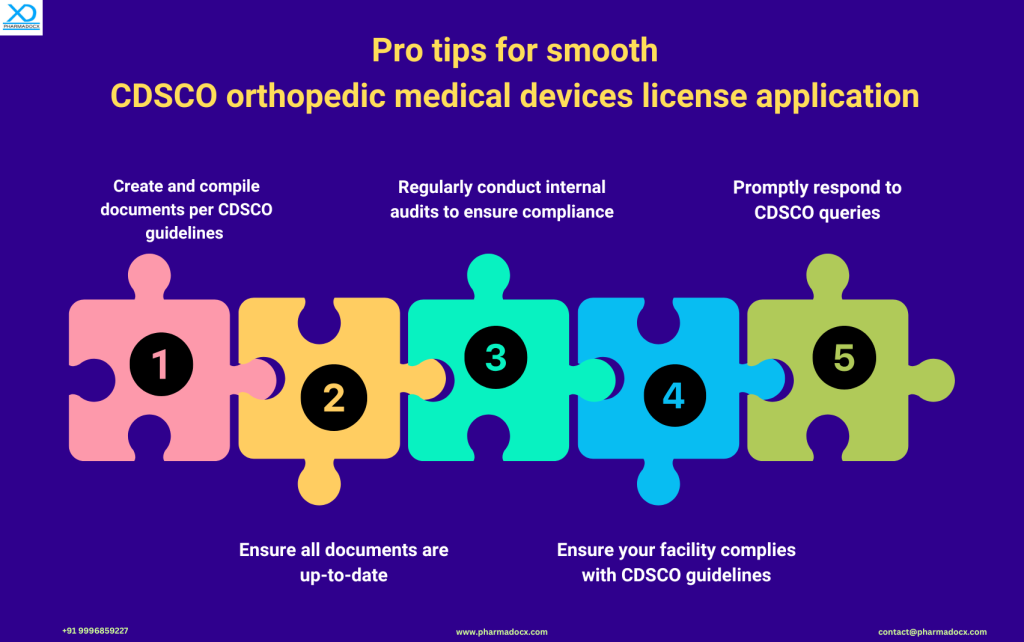
Common License Application Challenges & How to Overcome Them
Manufacturers and importers might face the following challenges while applying for their CDSCO license:
- Documentation Gaps: Ensuring all documents are up-to-date and meet CDSCO’s requirements.
- Delays in Query Resolution: Setting up a dedicated team to address CDSCO queries promptly.
- Inspection Hurdles: Regular internal reviews of facilities to ensure they meet CDSCO guidelines.
Validity of the CDSCO Orthopedic Medical Device Registration and License
CDSCO orthopedic medical device registration and license are valid indefinitely. However, to maintain the validity, the license retention fee has to be paid every 5 years.
How Can Pharmadocx Consultants Assist You in Securing the CDSCO Orthopedic Medical Devices License?
Pharmadocx Consultants offers a suite of services tailored for orthopedic and prosthetics device manufacturers:
- Expert Guidance: Assistance throughout the CDSCO medical device registration process.
- Mock Audits: Conducting pre-inspection audits to ensure readiness.
- Query Resolution: Our team will help you promptly address CDSCO queries.
- End-to-End Support: Comprehensive support from the initial application to license approval. We will provide support till you successfully secure the CDSCO medical device license.
- Learn More
The CDSCO registration for orthopedic and prosthetics devices ensures that healthcare professionals are equipped with the best tools for patient care. With the upcoming deadline, it’s crucial for manufacturers and importers to act swiftly. With expert partners like Pharmadocx Consultants, the registration journey will be streamlined and efficient for you.
Compliance with CDSCO regulatory guidelines is a testament of the manufacturer’s dedication to patient safety and product quality. Furthermore, staying compliant ensures trust in the brand and helps avoid potential regulatory repercussions. Seeking expert guidance for CDSCO registration for orthopedic medical devices? Drop an email at [email protected] or call/Whatsapp on 9996859227 to let us help you.





