Medical devices and IVDs are pivotal components of the healthcare sector. They are vital for effective healthcare delivery. Demonstration of safety and efficacy of these devices is crucial prior to marketing these products for use. To ensure improved outcome and patient safety, CDSCO has laid down several regulatory requirements and criteria. The companies selling these products in India need to abide by the CDSCO regulations. This blog explains what are medical devices and IVDs, the differences between them, and CDSCO regulations for them.
What are medical devices?
Medical devices are any apparatus, instrument, appliance, machine, implant, or software that can be used alone or in combination with other products to diagnose, alleviate, treat, or prevent diseases. They may come in direct contact with the patients.
Examples: syringes, catheters, hemodialysis machines, X-ray machines, CT scanners, hip prothesis, pacemakers, computer aided diagnostics, etc.
What are IVDs?
In vitro diagnostics (IVDs) are tools/devices used to test biological specimens (blood, tissues, or urine) to evaluate an individual’s health. They are used to detect the presence of a disease, infection, or medical condition. IVDS are used to perform tests on biological samples outside the human body. These devices cannot be used to treat patients but are used to determine the functioning of the body. Although these devices do not usually cause direct harm, IVDs pose risks if their use causes incorrect diagnoses. As opposed to medical devices or pharmaceuticals, these devices never come into direct contact with a person.
Examples: HIV tests, blood type identification tests, cancer screening devices, pregnancy self-test kits, urine test strips, etc.
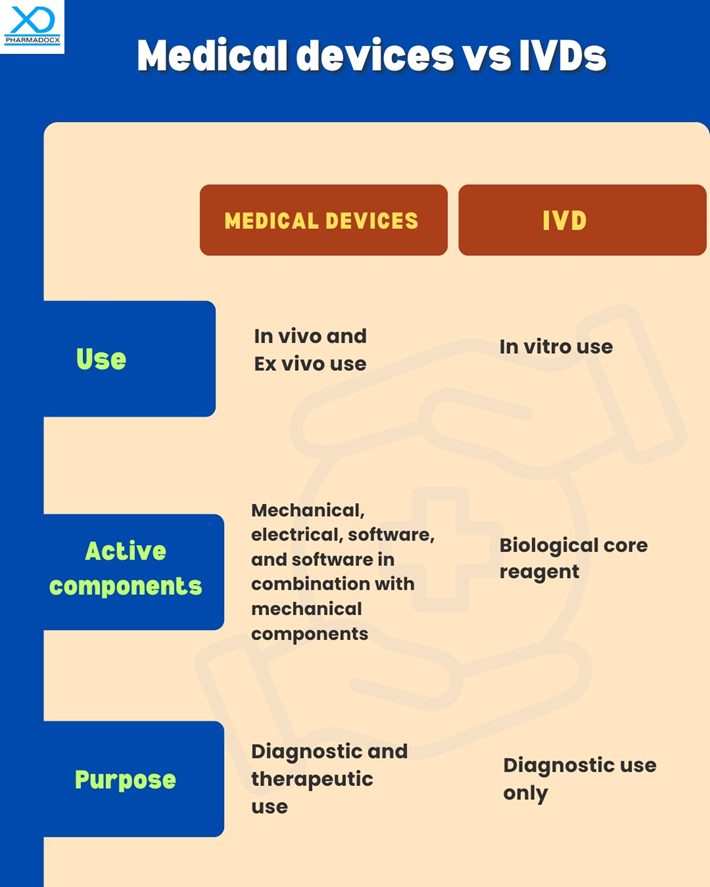
Key differences between medical devices and IVDs
Both medical devices and IVDs are key components of the healthcare system. Both are used for diagnostic purposes and are actively used by medical professionals to effectively deliver healthcare. However, there are some key differences between them.
CDSCO regulations for medical devices and IVDs
Central Drugs Standard Control Organisation (CDSCO) functions under the purview of Medical Devices Rules, 2017. The CDSCO is under the Ministry of Health and Family Welfare. The CDSCO regulations are in place to ensure patient safety and protect public health. All medical devices and IVDs are regulated in India under the provisions of the Medical Devices Rules, 2017. CDSCO regulations monitor the quality, safety, and efficacy of these healthcare components. These regulations ensure manufacturers focus on risk identification and reduction. The devices should perform as intended for effective healthcare delivery. CDSCO registration is mandatory to market these healthcare devices in India. CDSCO regulations for these devices guide and control their design, manufacturing, and labelling process. Additionally, CDSCO registration demonstrates the commitment of the manufacturer to regulatory compliance.
CDSCO definition
Medical devices: Any apparatus, appliance, or software that can be used alone or in combination for diagnostic and therapeutic use.
IVDs: IVDs are tools/substances intended to be used on specimens derived from humans for the diagnosis of any disease or disorder in human beings.
CDSCO classification
CDSCO has classified medical devices and IVDs for the ease of registration and licensing. The classification system per CDSCO regulations is based on risk level and intended use. The classification helps companies understand the registration process and regulatory requirements for their products.
Medical devices
- Class A medical devices are low-risk devices. Examples: gloves, hot water bags, bandages, walking aid, etc.
- Class B medical devices are low to moderate-risk devices. Examples: syringe, surgical instruments, nebulizers, etc.
- Class C medical devices are moderate-risk devices. Examples: X-ray machines, MRI machines, ventilators, cardiac stent, etc.
- Class D medical devices are high-risk devices. Examples: artificial joint, heart valve, etc.
In vitro diagnostics or IVDs
- Class A IVDs are low-risk devices. Examples: Prepared selective media, Clinical chemistry analyser, etc.
- Class B IVDs are low to moderate-risk devices. Examples: Urine test strips, pregnancy self-test kits, etc.
- Class C IVDs are moderate-risk devices. Examples: Rubella testing equipment, etc.
- Class D IVDs are high-risk devices. Examples: HIV blood donor screening, HIV blood test, etc.
Regulatory authorities for medical devices and IVDs
Different regulatory authorities are responsible for granting different licenses for different classes of medical devices and IVDs. Enforcement of rules has been divided between the following two arms of the CDSCO:
- Central Licensing Authority (CLA): CLA grants manufacturing license for Class C and D medical devices and IVDs. It grants import license for all classes of medical devices and IVDs. CLA grants test licences for manufacture or import of all classes of devices. Additionally, CLA conducts clinical performance evaluation and approves new devices. Furthermore, it oversees registration of notified bodies and laboratories that will carry out test or evaluation.
- State Licensing Authorities (SLA): SLA grants manufacturing license for sale or distribution of Class A and B medical devices and IVDs. It oversees sale, stock, exhibit or offer for sale or distribution of all classes of devices.
Import, manufacture, sale, distribution, and use of which IVD test kits and reagents are prohibited in India?
- Antibody detecting rapid diagnostic tests for routine diagnosis of malaria are prohibited
- Serodiagnostic test kits for diagnosis of tuberculosis are prohibited.
Important points to note regarding CDSCO regulations:
- In-vitro fertilization kit is regulated under medical device category and not IVD category.
- Instrument intend for research use only and not for in-vitro diagnosis using specimen collected from human or animal is not regulated under MDR 2017.
- CDSCO license remains valid indefinitely, if not cancelled by the regulatory body. However, a license retention fee has to be paid at specific intervals.
- The 5-year period for payment of license retention fees of an endorsement issued on a particular base license is calculated based on the issue date of the base license and not endorsement issued.
- Various supporting documents along with the application form will be required for CDSCO registration. The CDSCO has published the list of supporting documents required. The Pharmadocx Consultants team can help you prepare and collate all the necessary documents. CDSCO registration document preparation will be a breeze with Pharmadocx Consultants.
- The mode of CDSCO registration is online. SUGAM is the official portal for online application for CDSCO registration.
- Microbiological culture media and reagents used for food and water testing and not for disease diagnosis are not regulated under MDR, 2017.
- Specimen collection tubes are regulated under MDR, 2017, under the definition of “specimen receptacle”.
Still have questions regarding the CDSCO regulations for medical devices and IVDs?
Medical devices and IVDs are in high demand in India. Hence, stringent guidelines are in place to regulate them. Understanding the CDSCO regulatory requirements is tricky and difficult. Armed with in-depth knowledge of the CDSCO regulations, we shall be glad to answer your queries. With over 27 years of experience and having served over 600 clients, we are seasoned with the CDSCO regulatory requirements. Pharmadocx Consultants can help you secure CDSCO manufacturing, import, and test licenses. Simply send your query to [email protected] or call/Whatsapp on 9996859227 and we will try to resolve it.





