Medical devices are the backbone of the healthcare sector. These devices are used to perform real time diagnosis of various diseases/conditions as well as treat and monitor them. India is set to become a global medical device manufacturing hub, with the aim of producing high-quality affordable medical devices. Notably, the Indian medical device industry is growing at an unprecedented scale. The Indian medical device sector aims to cater to the domestic demands. Additionally, it aims to export their products to the international market. Hence, medical device exporters/manufacturers planning to export notified medical devices need to secure the free sale certificate. In this blog, we have discussed how to apply for a free sale certificate for medical devices. Additionally, we have provided some tips to easily secure the medical device free sale certificate.
What is a free sale certificate?
Manufacturers planning to export notified medical devices need to secure a “free sale certificate” or “certificate for export”. The free sale certificate is issued by the national regulatory authority of the exporting country. This export document is mandatorily demanded for certain products. The certificate provides the assurance the goods mentioned in it are freely distributed and produced in the country that intends to export the them. Moreover, the certificate does prove the medical device is tested for safety and efficacy and is licensed for use in the country. Furthermore, the application for free sale certificate is to be sent to the CDSCO.
Need for a free sale certificate for medical devices
The free sale certificate is a mandatory document for exporting regulated/notified medical devices. The free sale certificate for medical devices is vital for the following reasons:
- Verification of the medical devices to be exported: The free sale certificate guarantees the authenticity of the medical devices to be exported. The importing country will strictly regulate all the drugs and medical devices entering the country. Thus, it will permit the import of those medical devices that are freely sold in the exporting country. Hence, the free sale certificate (FSC) assures the importing country that the device has been authorized for manufacturing in the country of origin.
- Mandatory document for securing permit to export to certain countries: Certain countries mandatorily demand the FSC for granting permit to import medical devices. Thus, exporters will have to mandatorily procure this certificate to market their devices in these countries.
- Validation of the manufacturer: The Free Sale Certificate is a definitive proof that the manufacturer holds a valid medical device manufacturing license in the country of origin. Thus, the importing country can be assured the manufacturer is licensed and authorised to manufacture the medical devices. Hence, the FSC validates the authenticity of the medical device manufacturer.
Who can apply for the medical device free sale certificate?
Any manufacturer having a valid manufacturing license can apply for the medical device free sale certificate from CDSCO.
Documents required for securing free sale certificate for medical devices
We have listed some of the vital documents required for applying for free sale certificate for medical devices.
- A copy of the valid medical device manufacturing license
- Cover letter stating the applicant’s name and address that should be duly signed and stamped by the organization’s head. Additionally, the cover letter should be written on the organization’s letterhead.
- List of products for which the FSC is being applied for
- The product permission letters for the products for which FSC is being applied for
- Letter of the concerned authority requesting for the certificate
- A notarized stamp paper stating no market complaint and adverse event reported against the medical device
- Copy of the authorisation letter or power of attorney, if required
The above list provides an overview of the documents required for applying for medical device free sale certificate. Feel free to contact us for a detailed list of necessary documents for the free sale certificate application. Additionally, we will help you prepare and collate the documents as per guidelines. Our document preparation service team will help you easily prepare all the necessary documents within the stipulated timeline. Furthermore, we will also provide a detailed checklist of documents for medical device FSC application for your easy reference.
How to apply for free sale certificate from CDSCO?
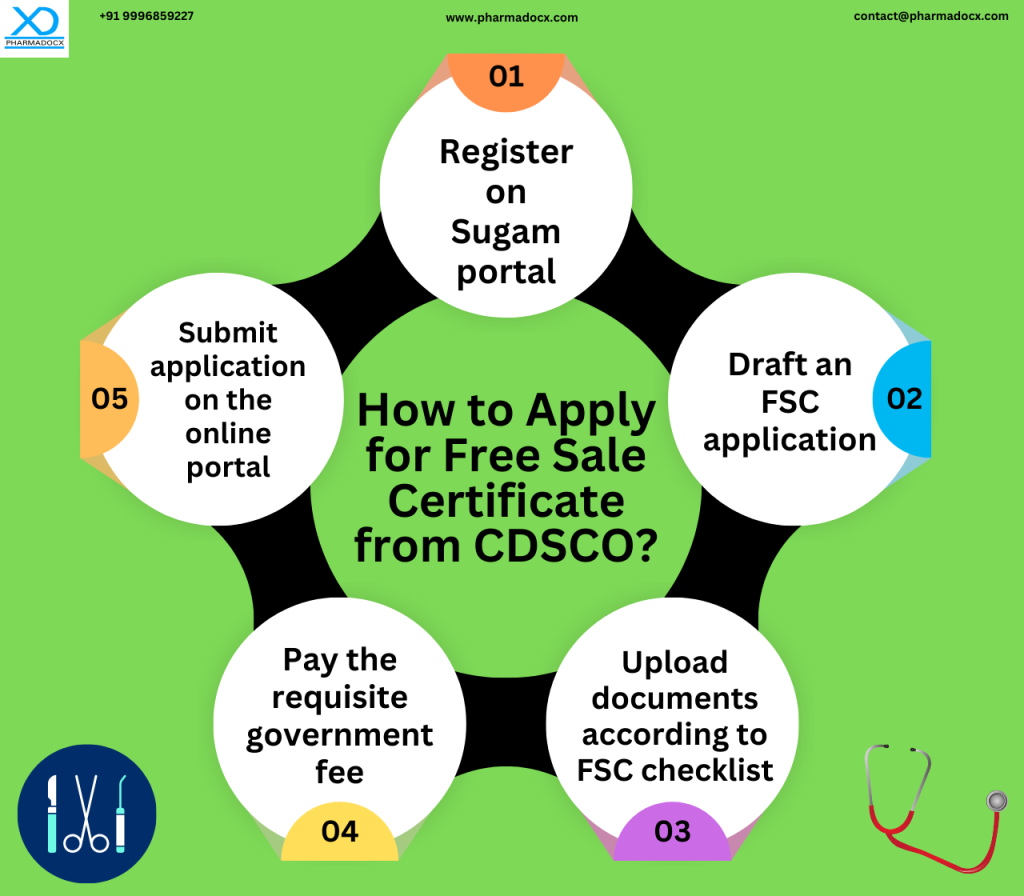
What is the validity of the free sale certificate for medical devices?
The free sale certificate will remain valid up to the validity of the medical device manufacturing license.
6 pro tips for easily securing the free sale certificate for medical devices
- A valid medical device manufacturing license is a mandatory requirement for obtaining free sale certificate for medical devices
- To apply for free sale certificate from CDSCO, the medical device must belong to the notified category.
- A manufacturer having no complaints against their devices can easily obtain the medical device FSC
- All the supporting documents must comply with the FSC checklist.
- A legal undertaking stating no market complaint or adverse event has been reported against the product is required
- The applicable government fee for securing the FSC must be paid
What is the fee and time required for obtaining the free sale certificate from CDSCO?
To apply for the free sale certificate, a fee of INR 1000 is required for each separate medical device. A period of 30 working days is required for securing the free sale certificate from CDSCO.
How will Pharmadocx Consultants help you secure the free sale certificate for medical devices?
At Pharmadocx Consultants, we are committed to streamline the medical device free sale certificate application process. We will help you secure the medical device FSC in a hassle-free manner so that you can easily start exporting your devices. Our FSC application service will ensure your product reaches foreign markets in a seamless manner.
- We will share the list of documents required for free sale certificate application. Additionally, we will help you prepare the documents and collate the documents per guidelines. Moreover, our document preparation team provides document drafting service. We will take care of the FSC application document preparation.
- Our team of experts will help you file the FSC application on the portal.
- Our team will keep track of the application status. We will provide real time update of the free sale certificate application progress.
- We will provide full support in case of any setbacks or queries. Our team will be there for you till you successfully secure the Free Sale Certificate.
Manufacturers planning to export medical devices are required to procure the FSC. Exporters require the FSC attesting the medical device is freely marketed and sold in India without any restrictions. Securing the free sale certificate for medical devices can be a cumbersome task. Avail our free sale certificate application service for a seamless and hassle-free application process. Our support does not end with filling the medical device free sale certificate application. The Pharmadocx Consultants team will provide full support till you successfully secure the free sale certificate from CDSCO. To start your medical device free sale certificate application process, drop an email at [email protected] or call/Whatsapp on 9996859227.