An implant card (IC) is a patient-specific document that accompanies an implantable medical device. It provides essential information on the person’s implanted medical device. It is required by Article 18 of the EU medical device regulations (MDR). Moreover, it must be provided by the manufacturer to the patient at the time of implantation. The card should provide key identification details, such as the device name, model, serial or lot number, and UDI. Additionally, the manufacturer’s name, address, and website should be provided. The card must also contain warnings, precautions, expected device lifetime, follow-up requirements, and any other safety information. It must be written in the official language(s) of the Member State, be understandable to laypersons, and be easily accessible. In this blog, we have focused on the EU MDR implant card requirements.
The purpose of the implant card under the EU MDR is to empower patients, enhance safety, and support traceability of implantable medical devices. Healthcare institutions are responsible for ensuring the card is delivered to the patient at the time of implantation. Compliance with implant card requirements is mandatory for compliance with EU MDR. However, certain implants, such as sutures and dental fillings, are exempt from EU MDR implant card requirements.
What is an implant card?
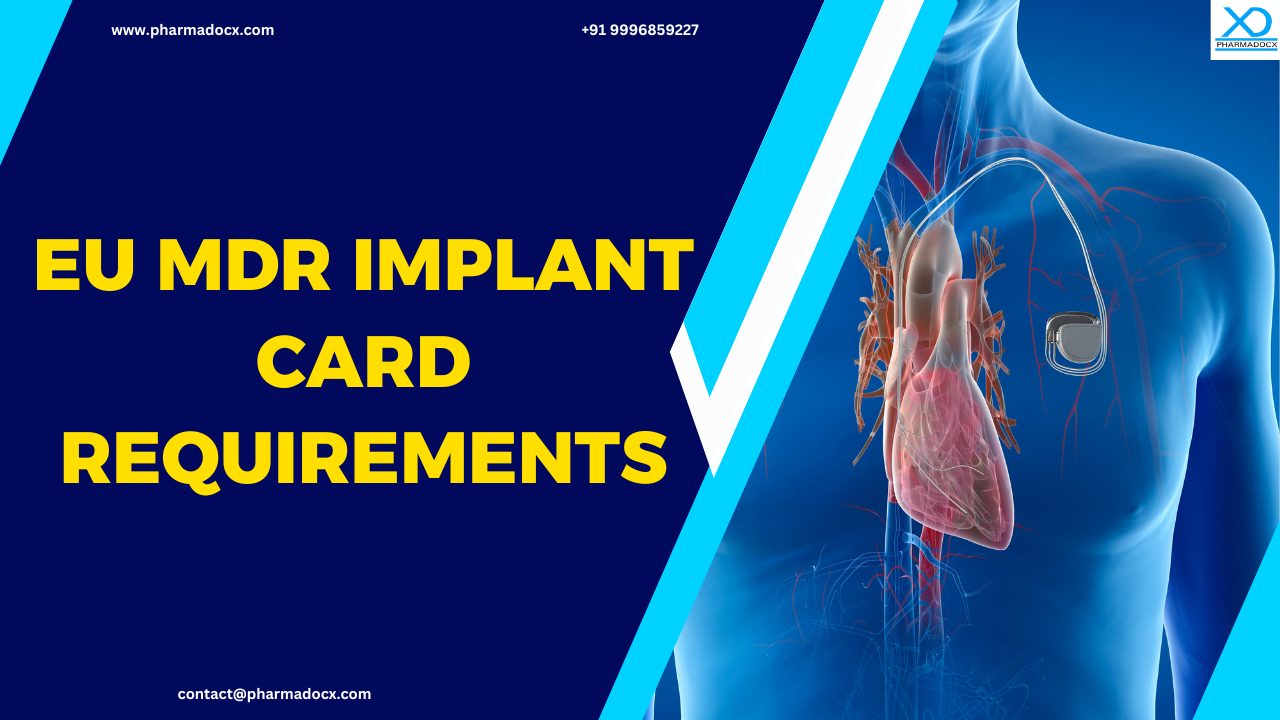
An implant card is a credit card-sized personalized document provided to patients who receive an implantable medical device. Implantable medical devices are surgically placed in the human body either permanently or temporarily to support functions of specific organs or tissues or deliver medication. The implant card contains essential information about the device to support safety, traceability, and emergency care.
The implant card contains details, such as the device’s name, manufacturer, and serial number, which are critical for patient safety and care, especially in medical emergencies. The card serves as an identification tool for the patient’s implanted medical devices. It allows healthcare professionals to quickly access vital information, such as whether the device is compatible with an MRI or requires specific treatment protocols.
4 Primary purposes of the implant card
- Patient safety and emergency care: The implant card enables patients to communicate critical device information to healthcare providers, especially in emergencies. It helps clinicians assess device compatibility with procedures like MRI, surgery, or diagnostics. Reduces risks from external influences (e.g., electromagnetic interference, environmental exposure). Hence, implant card requirements are essential for patient safety and emergency care.
- Device traceability and surveillance: It facilitates rapid identification of the implanted device using UDI, model, and serial number. Thus, implant card supports post-market surveillance, including adverse event reporting and field safety corrective actions. Furthermore, it enables regulatory authorities to trace devices during recalls or safety alerts.
- Patient empowerment and informed care: Implant cards provide patients with clear and understandable information about their implant. Healthcare providers can develop their follow up care based on the information provided. Furthermore, it builds confidence and transparency in medical device use.
- Regulatory compliance: Having the implant card in place is vital to comply with the implant card requirements under EU MDR Article 18, which mandates that manufacturers provide implant cards. Notably, healthcare institutions are required to deliver the card to the patient at the time of implantation. Furthermore, providing the implant card aligns with broader MDR goals of transparency, safety, and harmonization across Member States.
EU MDR implant card requirements
As per EU MDR implant card requirements, manufacturers of implantable medical devices must provide an implant card, containing essential device and safety information, to patients. The implant cards are designed to ensure patient safety, device traceability, and regulatory compliance.
EU MDR Article 18
Implant card requirements are mandated under Article 18 of EU MDR, which aims to:
- Enhance transparency and patient safety
- Ensure traceability of implantable devices
- Support emergency care, adverse event reporting, and device recalls
Implant card requirements: What should the implant card include?
- Device identification information: This section ensures traceability and supports post-market surveillance. This section should include device name, model, serial number or lot number, and Unique Device Identifier (UDI) (both UDI-DI and UDI-PI, if applicable).It enables quick identification during emergencies, recalls, or adverse event reporting.
- Manufacturer information: Manufacturer’s name, address, and website are to be included. This allows patients and healthcare professionals to contact the manufacturer or access updated device information. The aim is to facilitate communication and access to updated safety or performance data.
- Warnings and precautions: Critical safety information for patients and clinicians. This section should detail MRI compatibility of the device and electromagnetic interference as well as impact of exposure to heat, moisture, or chemicals. Additionally, contraindications for certain procedures have to be provided. This section is essential to prevent misuse or complications due to external influences or medical procedures.
- Expected lifetime and follow-up: Information about how long the device is expected to function and any required monitoring information. Recommendation for follow-up schedule or checks. This section helps patients and clinicians plan long-term care and device maintenance.
- Safe use instructions: Additional guidance to ensure the implantable device is used correctly and safely. Handling instructions and activity restrictions are provided. This section aims to reduce risk of misuse and enhances patient understanding.
Format and language requirements for the implant card
- Implant card format: The implant card maybe physical or digital. The physical card is usually of standard credit card dimensions (85.6 mm × 54 mm) and printed on sturdy material as well as are durable and portal. The card must include space for patient identity and implantation details (added by healthcare provider). It enables emergency responders and clinicians to identify the implant and patient quickly.
- Language and accessibility: The implant card must be written in the official language(s) of the Member State where the device is implanted. It must be understandable by a layperson and must be accessible rapidly, including via digital means (e.g., QR code, online portal). This requirement ensures patients can understand and access the information when needed.
Who must comply with the EU MDR implant card requirements?
Manufacturers of implantable medical devices, including orthopedic implants (e.g., hip, knee, spinal), cardiovascular implants (e.g., stents, pacemakers), dental implants, and neurological implants, are required to comply with the EU MDR implant card requirements.
What are the manufacturer responsibilities?
The manufacturer must provide the implant card with the device or at the time of implantation. They are required to ensure the card is accurate, legible, and up-to-date. Furthermore, include the implant card in the technical documentation and post-market surveillance plan. Additionally, the manufacturers must update the information on the card when necessary and make it available via their website.
Exemption from EU MDR implant card requirements
Devices that are not intended to remain in the body or are not considered “implantable” under MDR definitions are exempt from EU MDR implant card requirements. We have provided some examples of implants that are exempt from Article 18 requirements:
- Sutures
- Staples
- Dental fillings
- Dental braces
- Tooth crowns
- Screws, wedges, plates, wires, pins, clips, connectors
Notably, the European Commission may amend this list via delegated acts.
Practical applications for implant card
We have presented some of the practical applications of using an implant card.
- A patient with a pacemaker shows the implant card before undergoing an MRI.
- A surgeon checks the implant card before revision surgery.
- A regulator uses the UDI to trace a recalled batch.
Therefore, the EU MDR implant card requirements are designed to strengthen patient safety, improve device traceability, and ensure transparency across the healthcare system. By mandating implant cards, EU MDR empowers patients to manage their health more effectively. Additionally, with the help of implant cards, healthcare providers can deliver safe and informed care. The implant card also plays a vital role in post‑market surveillance, supporting rapid response in cases of recalls or adverse events. Not sure about the EU MDR requirements for your implantable medical devices? Email at [email protected] or call/Whatsapp on 9996859227 to contact our team of experts. We will be more than happy to help you identify the applicable regulatory guidelines.