CDSCO IVD Import License
Easily get CDSCO MD15 License for IVDs in the shortest time with least regulatory hurdles.
For importing IVDs, CDSCO MD 15 License is required. This is granted by Central authorities.
Expert consultation with industry leaders
Fast & hassle-free MD15 approval
Manufacturing Registration
Documentation that passes scrutiny the first time
Fast and accurate query replies
600+ Granted Approvals 27+ Years Experience
300+ Happy Clients












Central Drugs Standard Control Organisation (CDSCO) strictly regulates all in vitro diagnostic devices (IVDs) entering the Indian market. IVD importers are required to mandatorily secure the CDSCO IVD import license for importing IVDs into India. We at Pharmadocx Consultants provide end-to-end solutions to help you easily secure the CDSCO MD 15 license for importing IVDs.
What are IVDs?
In vitro diagnostic devices (IVDs) are used in patient’s homes, laboratories, or health care facilities. IVDs play an indispensable role in diagnosis, treatment selection, and patient monitoring. They are used to improve patient outcome by offering effective healthcare delivery. These devices form the cornerstone of modern healthcare, offering considerable benefits for patient management.
By definition, IVD are basically tests performed on samples, such as blood, urine, cerebral fluid, tissue, or other puncture fluids, obtained from the human body. The term “in-vitro” refers to “in glass” indicating IVD tests are performed outside living organisms. IVDs are used to collect and examine specimens obtained from the human body. Basically, they are used to understand the patient’s current state of health to determine the course of treatment or next course of action required. Thus, clinical decisions are supported by results obtained from IVDs.
In public health settings, IVDs are used for early detection and prevention of diseases. Hence, the IVDs should be able to provide fast, reliable, and correct diagnosis. Furthermore, IVDs are used in point of care settings, self-tests, and labs by trained professionals. Hence, IVDs are of paramount importance in various settings in the healthcare industry.
CDSCO Regulations for Importing IVDs into India
We have highlighted the vital role of IVDs in the healthcare industry for improved patient care and better patient management. IVDs have applications in multiple settings in the healthcare industry. India is still heavily import dependant for fulfilling its IVD needs. India imports large quantities of IVDs. Hence, CDSCO has developed strict regulations for IVDs entering the Indian market.
CDSCO stringently regulates IVDs being imported for quality, efficacy and safety. Compliance with all applicable Indian IVD regulations is mandatory to import IVDs into India. The apex regulatory body strictly enforces these regulations to ensure the Indian healthcare industry has access to the highest quality devices. Furthermore, it can take corrective actions in case of regulatory non-compliance. Hence, securing the CDSCO IVD import license is a mandatory requirement for importing IVDs into India. Importing IVDS without the necessary CDSCO license can lead to regulatory sanctions and fines.
Want to easily obtain the CDSCO IVD import license?
Contact us using the form below
CDSCO IVD Import License
Form MD 14
To import IVDs into India, an application has to be filed under Form MD 14.
MD 15 license
CDSCO MD 15 license is required for importing IVDs for sale and distribution in India.
Who can apply for the CDSCO IVD import license?
- Any wholesaler or importer who wants to import any IVDs for sale and distribution in India
- Any foreign IVD manufacturer who wants to supply IVDs in India. However, the foreign manufacturer will have to file the application via the authorised agent.
- An Indian authorized agent who has a valid wholesale license for the sale or distribution of IVDs.
- Any research institution in India who wants to import IVDs for clinical trials and research.
- Any hospital or healthcare provider who wants to import specialized IVDs or custom-made devices for their patients.
The import license applicant is required to possess a wholesale license (Form 20/21 B) or a registration certificate (Form 41/42).
Rules for Foreign IVD Manufacturers
Foreign IVD manufacturers who do not have local presence in India and are planning to sell their devices in India will have to appoint an authorized agent. The authorized agent is required to have a valid wholesale license (MD 42). Thus, the Indian authorized agent will file the CDSCO IVD import license application on behalf of the foreign manufacturer. Furthermore, the agent will act as a liaison between the foreign manufacturer and CDSCO.
Documents Required for CDSCO IVD Import License Application
Various supporting documents have to be prepared for demonstrating regulatory compliance of the IVDs. Accurate and correct documentation per guidelines is critical for smooth CDSCO regulatory approval. We have listed the documents necessary for CDSCO MD 15 license application.
- Notarized copy of overseas manufacturing site or plant registration in the country of origin issued by the competent authority
- Self-attested copy of valid manufacturing license demonstrating the device is manufactured in accordance with the regulatory requirements of the country of origin.
- Plant master file
- Device master file
- Notarized copy of ISO 13485 certificate of the actual IVD manufacturer
- Duly apostilled/notarized copy of Free Sale Certificate from National Regulatory Authority of country of origin
- Full Quality Assurance Certificate/CE type examination certificate/CE product quality assurance certificate
- Notarized Declaration of Conformity stating the device complies with applicable regulatory requirements and standards
- Notarized CE design certificate
- A copy of the latest inspection or audit report carried out by Notified bodies or the National Regulatory Authority within last 3 years
- Power of attorney, in case an authorized agent has to be appointed
- Constitution details of Indian authorized agent for foreign IVD manufacturers
For a detailed list of necessary supporting documents or for document preparation support, feel free to get in touch with us. We will prepare and compile the documents per CDSCO regulatory guidelines.
CDSCO IVD Import License Application: A Step-by-Step Guide
- Step 1 – Applicant registration: Register on the official CDSCO online portal, SUGAM.
- Step 2 – Document preparation: Prepare and compile all necessary supporting documents per CDSCO guidelines.
- Step 3 – CDSCO IVD import license application: Fill in the MD 14 application form with all the required details. Ensure accuracy and completeness.
- Step 4 – Online submission: Upload the duly filed in application form along with the necessary documents on the online portal. Next, pay the necessary fees for the CDSCO IVD import license application. Once you have successfully completed all the steps and submitted your application, you will receive an application number.
- Step 5 – CDSCO application review: The CDSCO will review the application for compliance with regulatory guidelines and verify the documents. They will check for any areas of non-conformities.
- Step 6 – CDSCO query response: CDSCO officials may raise queries asking for additional clarification and further documentation. It is important to promptly and appropriately respond to all queries. Response should be provided in a timely manner along with additional documents (if required) to support the response.
- Step 7 – Facility inspection and audit: The CDSCO may inspect the importing firm’s IVD facility to verify whether it meets all applicable quality and regulatory standards. CDSCO audits are conducted for ensuring regulatory compliance.
- Step 8 – MD 15 license approval and grant: CDSCO will evaluate all the information provided and check the response to the queries. If satisfied, they will approve the application. The CDSCO IVD import license will be granted under MD 15. This license will permit you to import the IVD specified in your application for sale and distribution in India.
Validity of the CDSCO Import License
The CDSCO IVD import license remains valid indefinitely. However, a license retention fee has to be paid every 5 years to retain its validity.
Pharmadocx Consultants: Simplifying your CDSCO IVD Import License Application Journey
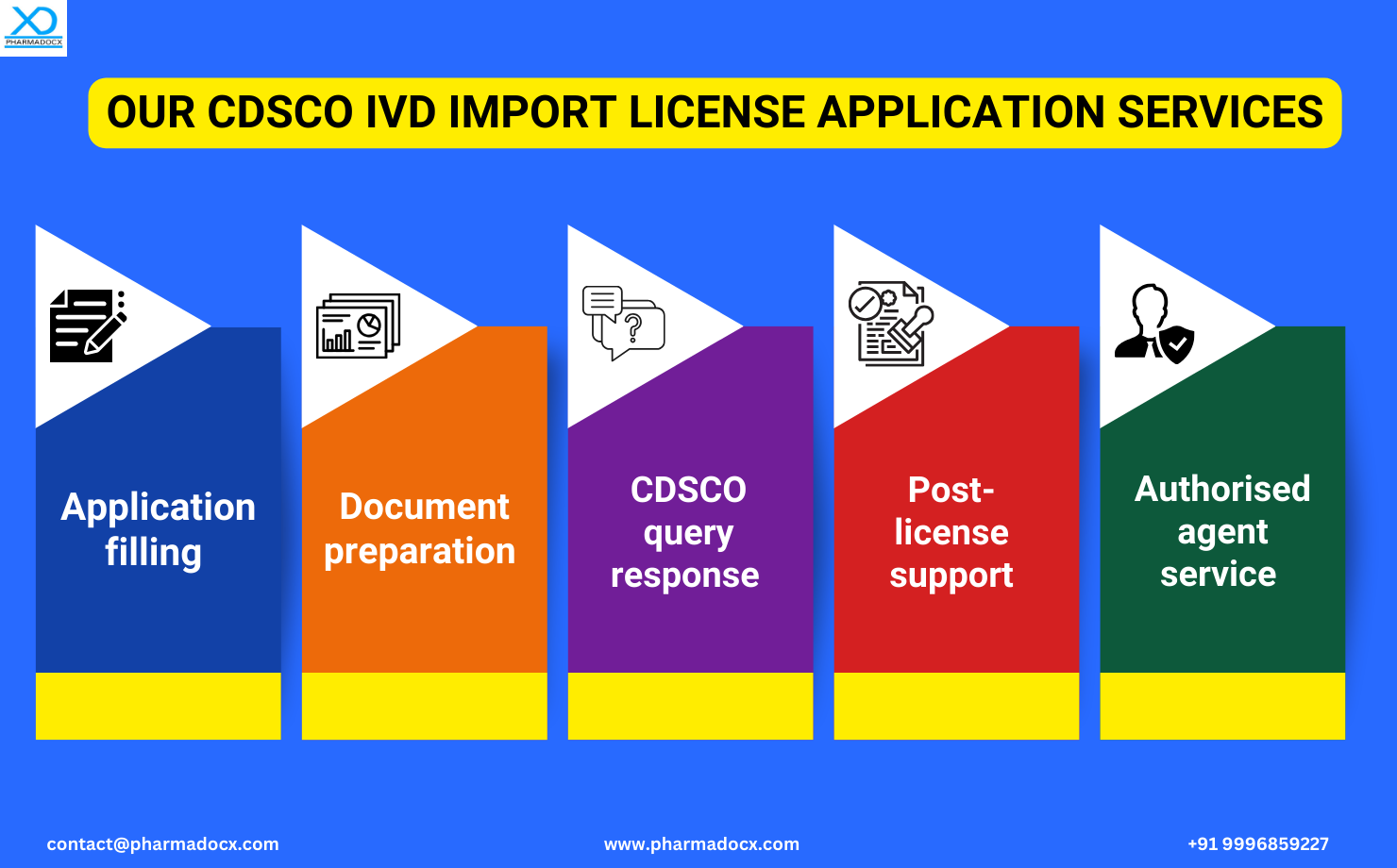
With over 27 years of experience and more than 600 clients, the Pharmadocx Consultants team has extensive knowledge of the CDSCO IVD regulations. We will leverage our expertise and industry knowledge to help you easily secure the CDSCO IVD import license. Drop an email at [email protected] or call/Whatsapp on 9996859227 to get started.
Let's Talk!
We'd love to hear from you! Whether you have questions about our pharmaceutical plant setup consultation services or want to discuss a potential project, our team is here to help. Simply fill out the form below, and we'll get back to you as soon as possible. Alternatively, you can reach out to us directly using the phone number or email address listed on this page. We look forward to connecting with you!
Phone / Whatsapp
Address
- Head Office - Opposite Dewan Mill, Old D.C. Road Sonepat - 131001 Haryana, India
- Registered Office - Netaji Subhash Place, Delhi, 110034


